Principal Investigators
-
Yukie Nagai, Ph.D.

Yukie Nagai, Ph.D.
Principal Investigator
Computation
Project Professor
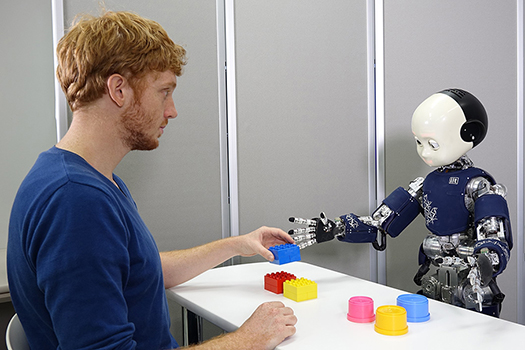
Understanding and assisting human intelligence through cognitive developmental robotics
Research
Human infants acquire diverse cognitive functions within the first few years of life. Although the dynamics of cognitive development have been increasingly analyzed, the underlying neural mechanisms are still not fully understood. We aim to clarify these mechanisms from a constructive perspective based on the embodied predictive processing theory, which extends general principles of brain function. Predictive processing is a framework in which internal models are updated and actions are generated to minimize prediction errors—the differences between bottom-up sensory inputs and top-down predictions. By incorporating embodiment into this framework, we explore how not only brain refinement but also sensorimotor development and social interaction influence cognition. We implement neural network models grounded in this theory into robots and allow them to learn from varied sensorimotor experiences to examine how and to what extent developmental continuity and diversity can be accounted for. The findings are further applied to assistive technologies for individuals with developmental disorders, aiming to promote self-understanding and support the design of an inclusive society.
Publications
Aude Billard, Alin Albu-Schaeffer, Michael Beetz, Wolfram Burgard, Peter Corke, Matei Ciocarlie, Ravinder Dahiya, Danica Kragic, Ken Goldberg, Yukie Nagai, and Davide Scaramuzza, “A roadmap for AI in robotics,” Nature Machine Intelligence, 7:818-824, June 2025.
Tsujita, M., Homma, M., Kumagaya, S., and Nagai, Y. Comprehensive intervention for reducing stigma of autism spectrum disorders: Incorporating the experience of simulated autistic perception and social contact. PLoS ONE, 18(8):e0288586, 2023.
Philippsen, A., Tsuji, S., and Nagai, Y. Quantifying developmental and individual differences in spontaneous drawing completion among children. Frontiers in Psychology, 13:783446, 2022.
Philippsen, A., Tsuji, S., and Nagai, Y. Simulating Developmental and Individual Differences of Drawing Behavior in Children Using a Predictive Coding Model. Frontiers in Neurorobotics, 16:856184, 2022.
Seker, M. Y., Ahmetoglu, A., Nagai, Y., Asada, M., Oztop, E., and Ugur, E. Imitation and mirror systems in robots through Deep Modality Blending Networks. Neural Networks, 146:22-35, 2022.
Friston, K., Moran, R. J., Nagai, Y., Taniguchi, T., Gomi, H., and Tenenbaum, J. World model learning and inference. Neural Networks, 144:573-590, 2021.
Lanillos, P., Oliva, D., Philippsen, A., Yamashita, Y., Nagai, Y., and Cheng, G. A review on neural network models of schizophrenia and autism spectrum disorder. Neural Networks, 122:338-363, 2020.
Nagai, Y. Predictive learning: its key role in early cognitive development. Philosophical Transactions of the Royal Society B: Biological Sciences, 374(1771):20180030, 2019.
Horii, T., Nagai, Y., and Asada, M. Modeling Development of Multimodal Emotion Perception Guided by Tactile Dominance and Perceptual Improvement. IEEE Transactions on Cognitive and Developmental Systems, 10(3):762-775, 2018.
Baraglia, J., Nagai, Y., and Asada, M. Emergence of Altruistic Behavior Through the Minimization of Prediction Error. IEEE Transactions on Cognitive and Developmental Systems, 8(3):141-151, 2016.
Biography
Yukie Nagai received her Ph.D. in Engineering from Osaka University in 2004. Following her doctoral studies, she held research positions at the National Institute of Information and Communications Technology, Bielefeld University, and Osaka University. Since 2019, she has served as a Project Professor at the International Research Center for Neurointelligence (IRCN) at the University of Tokyo, where she leads the Cognitive Developmental Robotics Laboratory. Her research explores the developmental mechanisms of human social cognition through computational approaches. In recognition of her contributions, she was selected as one of the Analytics Insight “World’s 50 Most Renowned Women in Robotics” in 2020, one of the IEEE IROS “35 Women in Robotics Engineering and Science” in 2022, one of the Forbes JAPAN “Women In Tech 30”, among other honors. She is also the principal investigator of two JST CREST projects: “Cognitive Mirroring” since 2016 and “Cognitive Feeling” since 2021. Her concurrent roles include Project Professor at both the Next Generation Artificial Intelligence Research Center and the Center for Coproduction of Inclusion, Diversity, and Equity at the University of Tokyo; Visiting Professor at the Center for Human Nature, Artificial Intelligence, and Neuroscience at Hokkaido University; and Associate Member of the Science Council of Japan.
-
Naohiro Okada, M.D., Ph.D.

Associate Professor, Graduate School of Medicine (Continues as IRCN Associate Professor (concurrent appointment))
Neuroimaging of Psychiatric Disorders and Adolescent Development
Research
Functional magnetic resonance imaging (fMRI) facilitates the quantitative assessment of brain structure and function in vivo. Our laboratory conducts human brain MRI studies in healthy adolescents and in patients with psychiatric disorders to understand the mechanisms linking them. Recently, we have reported a new brain-based classification related to cognitive and social functions, using a large-scale data set from a multi-site consortium. Current research is directed toward elucidating psychiatric disorders by revealing their neurobiological substrates and developing biomarkers for clinical applications. In addition, given the importance of international collaboration among MRI researchers, of connecting human and animal research, and of applying machine learning and AI techniques..., we collaborate extensively with multi-disciplinary researchers.
Publications
Okada N, Yahata N, Koshiyama D, Morita K, Sawada K, Kanata S, Fujikawa S, Sugimoto N, Toriyama R, Masaoka M, Koike S, Araki T, Kano Y, Endo K, Yamasaki S, Ando S, Nishida A, Hiraiwa-Hasegawa M, Edden RAE, Sawa A, Kasai K. Longitudinal trajectories of anterior cingulate glutamate and subclinical psychotic experiences in early adolescence: the impact of bullying victimization. Mol Psychiatry. 2024 Apr;29(4):939-950. doi: 10.1038/s41380-023-02382-8.
Okada N, Fukunaga M, Miura K, Nemoto K, Matsumoto J, Hashimoto N, Kiyota M, Morita K, Koshiyama D, Ohi K, Takahashi T, Koeda M, Yamamori H, Fujimoto M, Yasuda Y, Hasegawa N, Narita H, Yokoyama S, Mishima R, Kawashima T, Kobayashi Y, Sasabayashi D, Harada K, Yamamoto M, Hirano Y, Itahashi T, Nakataki M, Hashimoto RI, Tha KK, Koike S, Matsubara T, Okada G, van Erp TGM, Jahanshad N, Yoshimura R, Abe O, Onitsuka T, Watanabe Y, Matsuo K, Yamasue H, Okamoto Y, Suzuki M, Turner JA, Thompson PM, Ozaki N, Kasai K, Hashimoto R. Subcortical volumetric alterations in four major psychiatric disorders: a mega-analysis study of 5604 subjects and a volumetric data-driven approach for classification. Mol Psychiatry. 2023 Dec;28(12):5206-5216. doi: 10.1038/s41380-023-02141-9.
Okada N, Yamamoto Y, Yahata N, Morita S, Koshiyama D, Morita K, Sawada K, Kanata S, Fujikawa S, Sugimoto N, Toriyama R, Masaoka M, Koike S, Araki T, Kano Y, Endo K, Yamasaki S, Ando S, Nishida A, Hiraiwa-Hasegawa M, Yokoyama C, Kasai K. Birth order and prosociality in the early adolescent brain. Sci Rep. 2021 Nov 8;11(1):21806. doi: 10.1038/s41598-021-01146-0.
Okada N, Yahata N, Koshiyama D, Morita K, Sawada K, Kanata S, Fujikawa S, Sugimoto N, Toriyama R, Masaoka M, Koike S, Araki T, Kano Y, Endo K, Yamasaki S, Ando S, Nishida A, Hiraiwa-Hasegawa M, Kasai K. Abnormal asymmetries in subcortical brain volume in early adolescents with subclinical psychotic experiences. Transl Psychiatry. 2018 Nov 28;8(1):254. doi: 10.1038/s41398-018-0312-6.
Okada N, Fukunaga M, Yamashita F, Koshiyama D, Yamamori H, Ohi K, Yasuda Y, Fujimoto M, Watanabe Y, Yahata N, Nemoto K, Hibar DP, van Erp TG, Fujino H, Isobe M, Isomura S, Natsubori T, Narita H, Hashimoto N, Miyata J, Koike S, Takahashi T, Yamasue H, Matsuo K, Onitsuka T, Iidaka T, Kawasaki Y, Yoshimura R, Watanabe Y, Suzuki M, Turner JA, Takeda M, Thompson PM, Ozaki N, Kasai K, Hashimoto R. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry. 2016 Oct;21(10):1460-6. doi: 10.1038/mp.2015.209.
Biography
I obtained an M.D. degree from the Faculty of Medicine of the University of Tokyo in 2004. After a junior residency, I started psychiatry training at the University of Tokyo Hospital in 2006 and then worked as a psychiatrist at Tokyo Metropolitan Matsuzawa Hospital from 2007 to 2012. I became an Assistant Professor at the University of Tokyo Hospital in 2012 and concurrently entered the Graduate School of Medicine of the University of Tokyo earning a Ph.D. degree in 2017, before joining IRCN in 2019 where I manage the human fMRI scanner facility. At the same time, I am continuing research on the neuroimaging of psychiatric disorders and adolescent development.
-
Zenas C. Chao

Research
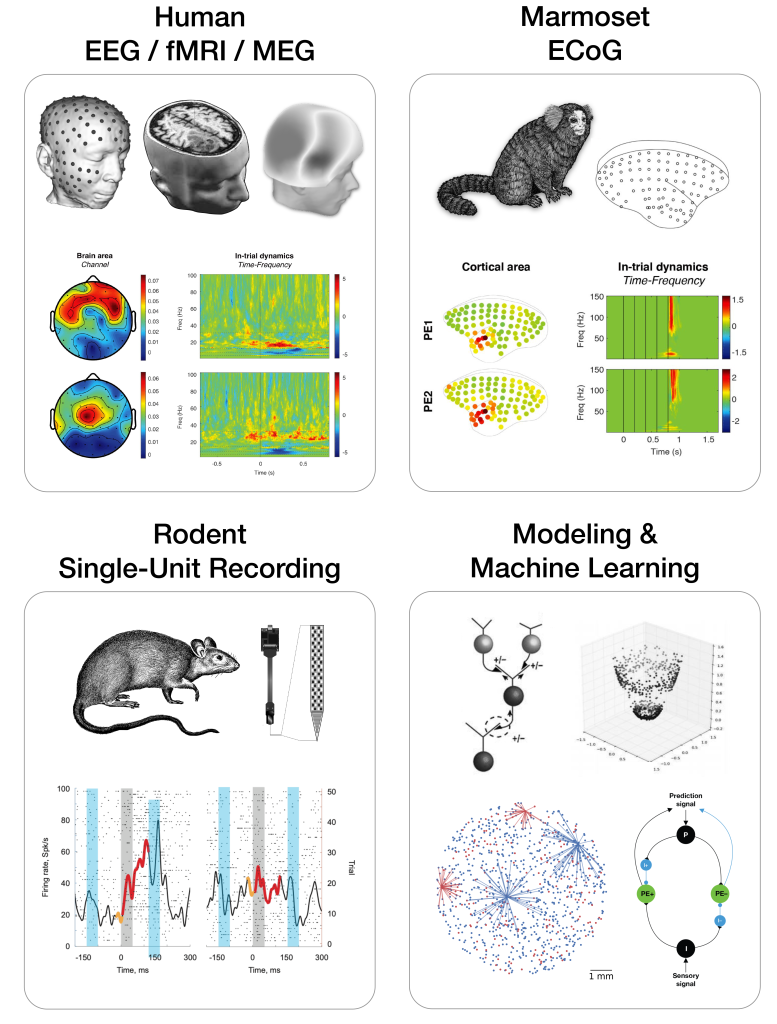
The predictive coding theory proposes that the brain continuously generates and updates predictions of sensory information at multiple levels of abstraction, and emits prediction-error signals when the predicted and actual sensory inputs differ. This theory offers a comprehensive framework for understanding perception, action, and internal processes such as creativity. The current focus of my lab’s research includes:
Predictive Coding Circuits: Our goal is to unravel the complex networks of microcircuits and macrocircuits essential to predictive coding across different hierarchical levels and sensory domains. Our research spans multiple species—from rodents and marmosets to humans—and combines experimental results from these varied subjects with computational modeling to develop comprehensive insights.
Neural Markers for Prediction-Related Psychiatric Disorders: A key focus of our research is to identify mechanistic neural markers associated with psychiatric disorders characterized by prediction anomalies, such as autism spectrum disorder. Understanding these neural foundations is essential for comprehending the conditions and could lead to high-resolution diagnoses and targeted interventions.
Predictive Coding in Creativity: We also explore the role of predictive coding in creativity potential, which we define as the brain’s capacity to generate novel and useful ideas for upcoming tasks. Our goal is to provide mechanistic insights into this complex process, linking creativity directly to its neural circuit implementation.
Creativity Augmentation for Real-World Applications: One ambition of our research is to develop a closed-loop system that enhances creativity potential in practical, real-world applications. This system has the potential to transform fields that rely on creative problem-solving by providing innovative tools and methodologies to boost innovation.
Publications
Chao ZC, Komatsu M, Matsumoto M, Iijima K, Nakagaki K, Ichinohe N (2024). Diverse configurations of erroneous predictive coding across brain hierarchies in a non-human primate model of autism spectrum disorder. Communications Biology, 7(1), 851.
Huang YT, Wu CT, Fang YM, Fu CK, Koike S, Chao ZC (2024). Crossmodal hierarchical predictive coding for audiovisual sequences in human brain. Communications Biology, 7(1), 965.
Chao ZC, Komatsu M, Matsumoto M, Iijima K, Nakagaki K, Ichinohe N (2024). Diverse configurations of erroneous predictive coding across brain hierarchies in a non-human primate model of autism spectrum disorder. Communications Biology, 7, 851.
Kern FB, Chao ZC (2023). Short-term neuronal and synaptic plasticity act in synergy for deviance detection in spiking networks. PLOS Computational Biology, 19(10), e1011554.
Chao ZC, Huang YT, Wu CT (2022). A quantitative model reveals a frequency ordering of prediction and prediction-error signals in the human brain. Communications Biology, 5(1), 1076.
Chao ZC, Takaura K, Wang L, Fujii N, Dehaene S (2018). Large-scale cortical networks for hierarchical prediction and prediction error in the primate brain. Neuron, 100(5), 1252-1266.
Chao ZC, Nagasaka Y, Fujii N (2015). Cortical network architecture for context processing in primate brain. Elife, 4, e06121.
Biography
Zenas Chao has a deep fascination with the human mind and the development of machines that mimic human intelligence. He earned B.S. degrees in Life Science and Chemistry in Taiwan before pursuing Biomedical Engineering at Georgia Institute of Technology, USA. During his Ph.D., he grew neurons in petri dishes and interfaced them with robots, demonstrating that machines equipped with an artificial organic brain can learn purposeful behaviors. Subsequently, he relocated to Japan, where he worked as a Research Scientist at the RIKEN Brain Science Institute, an Assistant Professor at the National Institute for Physiological Sciences, and a Junior Associate Professor at Kyoto University. His work in Japan focused on decoding brain signals from humans and monkeys to facilitate brain-controlled robots and computers. In 2019, Chao joined the International Research Center for Neurointelligence (IRCN) at the University of Tokyo. Here, he applies his research experience in silico, in vitro, and in vivo, to explore neural implementations of predictive coding, which is considered by many as a grand unified theory of cognition, and to investigate the neural mechanisms underlying creativity, a pivotal component of human intelligence.
-
Mayumi Kimura, Ph.D.

Mayumi Kimura, Ph.D.
Administrative Director
Project Professor
Think Global, Act Local – to build up interdisciplinarity and transborder approaches requisite for WPI research
Activity Summary
On top of creating an international research environment, one of important missions as a WPI center is the promotion of interdisciplinary and transborder research activities. IRCN is a research institution to find out “What is human intelligence?”. Our intellect seems develop not only by accumulation of academic knowledge, but also its emotional maturity would be added through exposure to a variety of art and culture. For example, to understand a unique Japanese intellect, having international and interdisciplinary background must be helpful. Establishing an individual personality is also very human like, which will be though feasible in future to understand it through computational analyses. Such a novel multidisciplinary challenge is requisite for WPI. “Think global, act local” is a business concept, but it is also applicable among researchers’ community. Isn't it exciting to think, new ideas of human intelligence will be spreading out from the UTokyo Hongo campus to the rest of world? Based on this policy, thinking globally and acting locally, I would dedicate my long-term scientific career to foster the next generation research and leaders.
Major achievements
Karamihalev S, Flachskamm C, Eren N, Kimura M, Chen A. (2019) Social context and dominance status contribute to sleep patterns and quality in groups of freely moving mice. Sci Rep 9:15190.
Gazea M, Patchev AV, Anderzhanova E, Leidmaa E, Pissioti A, Flachskamm C, Almeida OFX, Kimura M. (2018) Restoration of serotonergic homeostasis in the lateral hypothalamus rescues sleep disturbances induced by early-life obesity. J Neurosci 38:441 –451.
Kumar D, Dedic N, Flachskamm C, Voulé S, Deussing JM, Kimura, M. (2015) Cacna1c (Cav1.2) modulates EEG rhythm and REM sleep recovery. Sleep 38:1371-1380.
Jakubcakova V, Flachskamm C, Landgraf R, Kimura, M. (2012) Sleep phenotyping in a mouse model of extreme trait anxiety. PLoS ONE, 7:e40625.
Kimura M, Müller-Preuss P, Lu A, Wiesner E, Flachskamm F, Wurst W, Holsboer F, Deussing JM. (2010) Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol Psychiatry 15:154-165.
Kimura M, Kodama T, Aguila MC, Zhang SQ, Inoué S. (2000) Granulocyte-macrophage colony-stimulating factor (GM-CSF) modulates rapid eye movement (REM) sleep and non-REM sleep in rats. J Neurosci 20: 5544-5551.
Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. (1997) Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci USA 94:1023-1028.
Kimura M, Zhang SQ, Inoué S. (1996) Pregnancy-associated sleep changes in the rat. Am J Physiol 271:R1063-R1069.
Education/Academic background and major awards
Graduated from Kanazawa University (MSc in biology) and completed a Ph.D. in physiology at Tokyo Medical and Dental University, Japan. Mayumi Kimura was formally appointed at the Tokyo Medical and Dental University (1989-2003), The University of Tennessee, Memphis (1990-1993), The University of Texas Southwestern Medical Center at Dallas (1994-1995), Louisiana State University Pennington Biomedical Research Center (1995-1996), and Tulane University School of Medicine (1997-1998). From 2003 till 2018, she was appointed as a Research Group Leader at the Max Planck Institute of Psychiatry. During the last 35 years, she specialized in pre-clinical/basic sleep research with a focus on the neuro-humoral mechanisms of sleep-wake regulation. She advocated that sleep-wake behavior is under strong homeostatic control via immune challenge induction, gender-specific changes, and stress-driven sleep responses.
Back in Japan since June, 2018, Mayumi has shifted her scientific career from bench to administration. First appointed as Project Associate Professor at International Research Center for Neurointelligence (WPI-IRCN), The University of Tokyo (2018-2020), then as Professor at International Institute for Integrated Sleep Medicine (WPI-IIIS), University of Tsukuba, having served as IIIS Administrative Director (2021- March 2024). Currently, she is back again to WPI-IRCN as Project Professor and a head of management, namely Administrative Director (April, 2024-).
Awards
1999 Promising Scientist Award (the 4th), Society of Japanese Women Scientists
2001 Young Investigator Award (the 6th), Japanese Society of Sleep Research
2002 Biofuture Award, Institute of Biomaterials & Bioengineering, Tokyo Medical & Dental University
-
Takao Hensch, Ph.D.

IRCN Director / Principal Investigator / Project Professor
Human/Clinical
Professor of Neurology, Harvard Medical School, Boston Children’s Hospital
Professor of Molecular & Cellular Biology, Center for Brain Science, Harvard University
Critical Periods in Brain Development and their Clinical Applications
Research
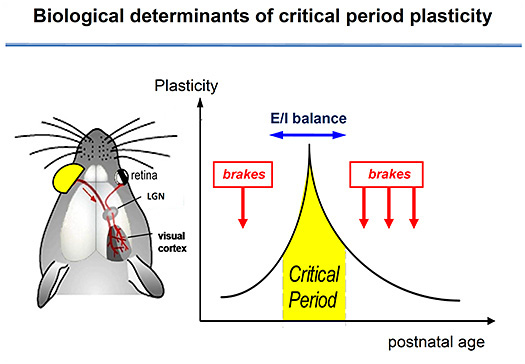
Early life experience shapes brain function – from motor skills to language and emotions. We explore the biological basis of these windows of opportunity and vulnerability and how they are derailed in mental illness. Integrating molecular, cellular and systems neuroscience, our work has revealed these “critical periods” are themselves plastic and reversible. Specific, inhibitory (GABA) circuits trigger their onset timing, while “brake”-like factors actively prevent circuit rewiring when they close. Translational research inspired by this work targets recovery from neurodevelopmental disorders, such as amblyopia, epilepsy and autism spectrum disorders, as well as the potential for recovery of function later in life.
Publications
Takesian AE, Bogart LJ, Lichtman JW, Hensch TK. (2018) Inhibitory circuit gating of auditory critical-period plasticity. Nature Neurosci. 21:218-227
Kobayashi Y, Ye Z, Hensch TK. (2015) Clock genes control cortical critical period timing. Neuron. 86:264-75.
Werker JF, Hensch TK. (2015) Critical periods in speech perception: new directions. Annu Rev Psychol. 66:173-96. Gogolla, N., Takesian, A.E., Feng, G., Fagiolini, M. & Hensch, T.K. (2014) Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron 83: 894-905.
Barkat, T.R., Polley, D.B. & Hensch, T.K. (2011) A critical period for auditory thalamocortical connectivity. Nature Neurosci. 14:1189-1194.
Morishita, H., Miwa, J.M., Heintz, N. & Hensch, T.K. (2010) Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 330:1238-1240.
Yazaki-Sugiyama, Y., Kang, S., Cateau, H., Fukai, T. & Hensch, T.K. (2009) Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature 462: 218-21.
Sugiyama, S., Di Nardo, A., Aizawa, S., Matsuo, I., Volovitch, M., Prochiantz, A. & Hensch, T.K. (2008) Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134: 508-520.
Hensch, T.K. (2005) Critical period plasticity in local cortical circuits. Nature Reviews Neurosci. 6: 877-888. Fagiolini, M., Fritschy, J-M., Löw, K., Möhler, H., Rudolph, U. & Hensch, T.K. (2004) Specific GABAA circuits for visual cortical plasticity. Science 303: 1681-1683.
Hensch, T.K & Stryker, M.P. (2004) Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science 303: 1678-1681. Fagiolini, M. & Hensch, T.K. (2000) Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404: 183-186.
Hensch, T.K., Fagiolini, M., Mataga, N., Stryker, M.P., Baekkeskov, S. & Kash, S.F. (1998) Local GABA circuit control of experience-dependent plasticity in the developing visual cortex. Science 282:1504-1508.Biography
After training with Drs J Allan Hobson at Harvard (AB), Masao Ito at UTokyo (MPH), Wolf Singer at the Max-Planck Institute for Brain Research (Fulbright fellow), and Michael P Stryker at the University of California San Francisco (PhD Neuroscience, 1996), Hensch helped to launch the RIKEN Brain Science Institute, initially as Lab Head for Neuronal Circuit Development then Group Director for Critical Period Mechanisms Research. He returned to Harvard in 2006 as joint Professor of Neurology and Molecular Cellular Biology, where he directs the NIMH Silvio Conte Center for Mental Health Research. Career honors include the Society for Neuroscience Young Investigator Award both in Japan (Tsukahara Prize, 2001) and the US (2005), the NIH Director's Pioneer Award (2007), Sackler Prize (2016) and co-Director of the CIFAR Child Brain Development network (2019). Hensch also serves on the editorial board of several prominent journals, including Neuron, Neural Development and Frontiers in Neural Circuits (Chief Editors).
-
Kantaro Fujiwara, Ph.D.

Data Science Core Core Manager
Associate Professor, Graduate School of Medicine (Continues as IRCN Associate Professor (concurrent appointment))
Computational Neuroscience, Neural Data Analysis and its Applications
Research
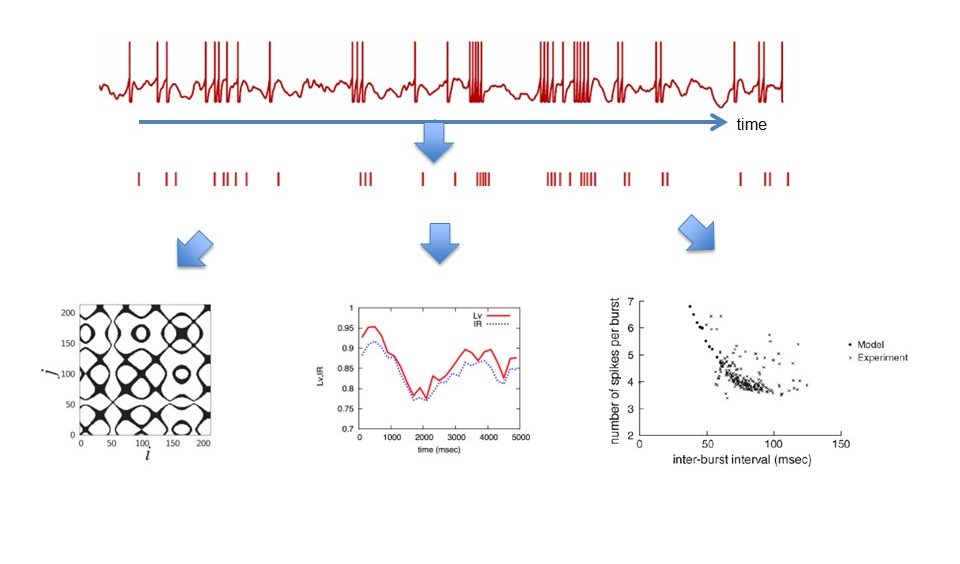
Neuroscience is an interdisciplinary research field that is linked by a wide variety of research fields. Theoretical approaches to neuroscience have helped to introduce new ideas and shape directions of neuroscience research. In modern neuroscience, theoretical neuroscience is assuming an increasingly important role due to big data obtained by experimental measurement and the recent development of artificial intelligence. In my current work, I focus on theories of neuronal data analysis and neural network modeling with the aim of bridging the gap between experimental and theoretical neuroscience studies. I also work on algorithmic study on brain-inspired machine learning.
Publications
Ziqiang Li, Kantaro Fujiwara, Gouhei Tanaka, “Dynamical Graph Echo State Networks with Snapshot Merging for Dissemination Process Classification”, Communications in Computer and Information Science, Vo. 1964, pp. 523-534, 2023.
Ziqiang Li, Kantaro Fujiwara, Gouhei Tanaka, “An Echo State Network-Based Method for Identity Recognition with Continuous Blood Pressure Data”, Lecture Notes in Computer Science, Vol. 14257, pp. 13-25, 2023.
Keita Koyama, Hiroyasu Ando, Kantaro Fujiwara, "Functional improvement in β cell models of type 2 diabetes using on-demand feedback control", AIP Advances, 13, 045317, 2023.
Ryota Nomura, Kantaro Fujiwara, Tohru Ikeguchi, "Superposed recurrence plots for reconstructing a common input applied to neurons", Physical Review E, 106, 034205, 2022.
Kotaro Kasahara, Yutaka Shimada, Kantaro Fujiwara, Tohru Ikeguchi, "Analysis on the mechanism of enhancing insulin secretion by TRPM2 channel in a pancreatic β-cell", Nonlinear Theory and Its Applications, IEICE, Vol. 12, No.3, pp. 500-511, 2021.
Ryota Nomura, Ying-Zong Liang, Kenji Morita, Kantaro Fujiwara, Tohru Ikeguchi, "Threshold-varying integrate-and-fire model reproduces distributions of spontaneous blink intervals", PLOS ONE, 13(10) e0206528, 2018.
Toshihiro Kobayashi, Yutaka Shimada, Kantaro Fujiwara, Tohru Ikeguchi, “Reproducing Infra-Slow Oscillations with Dopaminergic Modulation”, Scientific Reports, 7: 2411, 2017.
Biography
I received a Ph. D. in Information Science and Technology from the University of Tokyo. I studied computational neuroscience, especially the mathematical modeling of single neurons, and neural network modeling of learning and adaptation. As a postdoctoral researcher for JSPS, I studied data analysis of neural systems, including a theory for neural spike train analysis. As an Assistant Professor at Saitama University and Tokyo University of Science, I studied nonlinear mathematics and its applications. Current, I am aiming to bridge the gap between experimental and theoretical neuroscience. I also manage the data science core servers and software for the IRCN community.
-
Satoru Kondo, Ph.D.

Project Associate Professor
Mechanisms and Neuronal Circuits for Information Processing of Vision
Research
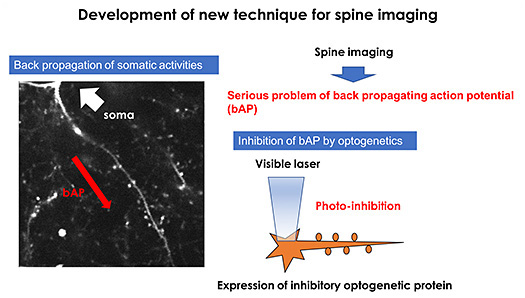
The brain is composed of neural circuits consisting of intricately connected neurons. I have been studying the synaptic organization of the neuronal circuits especially for vision. Visual information processed within the visual cortex is carried out at the level of single neurons and at the level of neuronal circuits. I am recording the large number of synaptic responses from single neurons and investigate how single neurons process synaptic inputs and generate output signals. By recording the synaptic inputs, I also investigate how neuronal information represented in individual single neurons is integrated. From these studies, I would like to understand the hierarchical neural circuits in which information of individual neurons is integrated to represent the external world.
Publications
Kondo S, Kiyohara Y and Ohki K: Response selectivity of the lateral posterior nucleus axons projecting to the mouse primary visual cortex. Front Neural Circuits 16:825735, 2022.
Kondo S, Yoshida T, Ohki K: Mixed functional microarchitectures for orientation selectivity in the mouse primary visual cortex. Nature Communications 7:13210, 2016.
Kondo S, Ohki K: Laminar differences in the orientation selectivity of geniculate afferents in mouse primary visual cortex. Nature Neuroscience 19:316-319, 2016.
Kubota, Y, Kondo, S, Nomura, M, Hatada, S, Yamaguchi, N, Mohamed, A A, Karube, F, Luebke, J, Kawaguchi, Y: Functional effects of distinct innervation styles of pyramidal cells by fast spiking cortical interneurons. eLIFE e07919, 2015.
Biography
1989 Graduated from the Faculty of Engineering, Kanazawa University
1991 Graduated from the Life Chemistry Course, Master Program, Graduate School of Engineering, Tokyo Institute of Technology.
1995 Graduated from the First Basic Medical Science Course, Doctoral Program, Graduate School of Medicine, The University of Tokyo.
1995 Ph.D. (doctorate in medicine) from the University of Tokyo.
1995-1997 Research Scientist at the Max Planck Institute for Biophysical Chemistry, Prof. Alain Marty's lab
1997-2004 Research Scientist at the RIKEN and Assistant Professor at the Institute of Physiological Sciences, Prof. Yasuo Kawaguchi's lab.
2004-2010 Lecturer at the Tokyo Medical and Dental University and at The University of Tokyo School of Medicine, Prof. Shigeo Okabe's lab.
2010-2018 Assistant Professor at the Kyushu University School of Medicine; Assistant Professor and Lecturer at The University of Tokyo School of Medicine, Professor Kenichi Ohki's lab
2018-present Specially Appointed Associate Professor at the IRCN, The University of Tokyo -
Hideki Ukai, Ph.D.

ES-Mouse/Virus Core Core Manager
Project Associate Professor
Development of Next-Generation Mammalian Genetics
Research
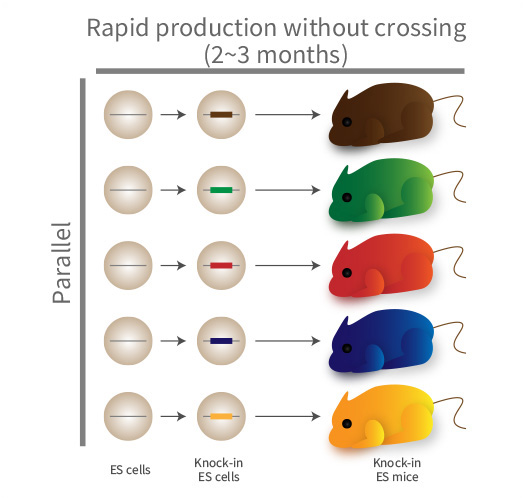
For elucidation of the mechanisms underlying higher-order biological phenomena, e.g., intelligence and mental disorders in humans, comprehensive identification and quantitative analysis of human gene-networks and cellular circuits are required. Reverse genetics is one of the powerful methods for this elucidation. However, mouse genetics is generally low throughput, due to the need for repeat crossing of chimeras/mosaics to produce genetically modified mice, and hence analyzing many genes/cells is difficult. Recently, we have established an efficient method to produce mutant mice without crossing. We are developing a method to elucidate human biological phenomena by applying the high-throughput production method of our mutant mice to genetically humanized mice in which mouse genes are replaced with human genes.
Publications
Ukai H, Sumiyama K, Ueda HR. Next-generation human genetics for organism-level systems biology. Current Opinion in Biotechnology. 2019. 58:137-145.
Ukai H, Kiyonari H, Ueda HR. Embryonic stem cell-mouse protocol to produce knock-in (KI) mice in a single generation. Nature Protocols 2017 DOI 10.1038/nprot.2017.110
*Ode KL, *Ukai H, *Susaki EA, Narumi R, Matsumoto K, Hara J, Koide N, Abe T, Kanemaki MT, Kiyonari H, Ueda HR. Knockout-Rescue Embryonic Stem Cell-Derived Mouse Reveals Circadian-Period Control by Quality and Quantity of CRY1. Molecular Cell 2017 Jan 5;65(1):176-190. * These authors contributed equally to this work.
Susaki EA, Ukai H, Ueda HR. Next-generation mammalian genetics toward organism-level systems biology. npj. Syst. Biol. Appl. 2017 3, article number 15.
Ukai H. and Ueda HR. Systems Biology of Mammalian Circadian Clock. Annual Review of Physiology 2010 72:579-603.
*Isojima Y, *Nakajima M, *Ukai H, Fujishima H, Yamada RG, Masumoto KH, Kiuchi R, Ishida M, Ukai-Tadenuma M, Minami Y, Kito R, Nakao K, Kishimoto W, Yoo SH, Shimomura K, Takao T, Takano A, Kojima T, Nagai K, Sakaki Y, Takahashi JS, Ueda HR. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc. Natl. Acad. Sci. USA. 2009 Sep 15;106(37):15744-9. * These authors contributed equally to this work.
*Ukai H, *Kobayashi TJ, Nagano M, Masumoto KH, Sujino M, Kondo T, Yagita K, Shigeyoshi Y, Ueda HR. Melanopsin-dependent photo-perturbation reveals desynchronization underlying the singularity of mammalian circadian clocks. Nature Cell Biology 2007 Nov;9(11):1327-34. * These authors contributed equally to this work.
*Sato TK, *Yamada RG, *Ukai H, Baggs JE, Miraglia LJ, Kobayashi TJ, Welsh DK, Kay SA, Ueda HR, Hogenesch JB. Feedback repression is required for mammalian circadian clock function. Nature Genetics 2006 Mar;38(3):312-9. * These authors contributed equally to this work.
Biography
I was a postdoctoral researcher at the National Institute of Radiological Sciences after earning a Ph.D. from the Graduate University for Advanced Studies. I developed the technologies for genome-scale analysis and discovered a new oncogenic mutation by using the technologies. Next, at the RIKEN CDB, I developed technologies for cellular-level systems biology using a circadian clock system as a model system of a complex phenomenon and elucidated the mechanism of the complex phenomenon such as the singularity behaviour of the circadian clock. After moved to the RIKEN QBiC, I started to develop the technologies toward organism-level systems biology to elucidate the mechanism of the higher-order biological phenomena, and have established an efficient method to produce mutant mice within a single generation. In my current position, by using my technology, I provide mutant mice and virus for researchers and also develop special mouse lines intended as common resources for the IRCN.
-
Takamitsu Watanabe

Takamitsu Watanabe
Principal Investigator
Human/Clinical
Professor
Network dynamics behind diverse intelligence
Research
Human cognition is dynamic, and its underlying brain activity is unstable. Thus, neural fluctuations should be essential for human intelligence and its development, while atypical brain dynamics should underpin neuropsychiatric conditions, including autism, schizophrenia and epilepsy. We pursue this framework and investigate biological mechanisms that link large-scale brain network architecture, global/local neural dynamics and typical/atypical cognition. We aim to utilise this research to understand diverse human and artificial intelligence.
Publications
Watanabe, T. & Yamasue, H. Noninvasive reduction of neural rigidity alters autistic behaviors in humans. Nature Neurosci. 28, 1348–1360 (2025).
Watanabe, T., Inoue, K., Kuniyoshi, Y., Nakajima, K. & Aihara, K. Comparison of Large Language Model with Aphasia. Advanced Sci. e2414016 (2025).
Watanabe, T. Causal roles of prefrontal cortex during spontaneous perceptual switching are determined by brain state dynamics. eLife 10, e69079 (2021).
Watanabe, T., Rees, G. & Masuda, N. Atypical intrinsic neural timescale in autism. eLife 8, e42256 (2019).
Watanabe, T. & Rees, G. Brain network dynamics in high-functioning individuals with autism. Nature Commun. 8, 16048 (2017).
Watanabe, T. et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain 138, 3400–3412 (2015).
Watanabe, T., Masuda, N., Megumi, F., Kanai, R. & Rees, G. Energy landscape and dynamics of brain activity during human bistable perception. Nature Commun. 5, 4765 (2014).
Watanabe, T. et al. Two distinct neural mechanisms underlying indirect reciprocity. Proc National Acad Sci. 111, 3990–3995 (2014).
Watanabe, T. et al. Mitigation of Sociocommunicational Deficits of Autism Through Oxytocin-Induced Recovery of Medial Prefrontal Activity: A Randomized Trial. JAMA Psychiat. 71, 166–175 (2014).
Watanabe, T. et al. A pairwise maximum entropy model accurately describes resting-state human brain networks. Nature Commun. 4, 1370 (2013).
Biography
After medical training in The University of Tokyo (M.D., 2007) and its hospital, I completed a Ph.D. in the lab of Professor Yasushi Miyashita in the same university as a JSPS Research Fellow (2013). I conducted post-doctoral research with Professor Geraint Rees in University College London under JSPS and Marie-Curie Research Fellowships, respectively. In 2018, I was appointed Deputy Team Leader for the Miyashita lab in the RIKEN Center for Brain Science. I launched my lab in IRCN at UTokyo as an Associate Professor (UTokyo Excellent Young Researcher Program) in April 2020. In 2025, I was promoted to Professor in IRCN at UTokyo. Other career awards include the NeuroCreative Award and RIKEN Excellent Achievement Award.
-
Kazuo Emoto, Ph.D.

IRCN Deputy Director / Principal Investigator
Development
Professor
Department of Biological Sciences, Graduate School of Science, The University of TokyoNeuronal Morphogenesis in Development and Disease
Research
The human brain receives, processes, stores, and transmits complex information with great fidelity. The neuronal network that underlies these functions is comprised of an estimated 1011neurons linked by over 1014 synaptic connections between structurally and functionally different neurites, axons and dendrites. Precise pattering of dendrites as well as axons is essential for correct wiring and function of neural circuits. We combine genetics, imaging, and biochemical approaches to investigate the interplay between genetic and epigenetic control of neural morphogenesis, and deduce the functional importance of these regulatory systems in disease etiology, using the fruitfly and mouse as research models.
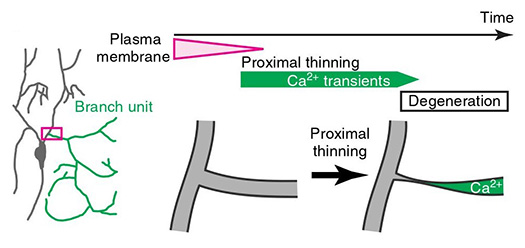
Publications
Yoshino J, Morikawa R, Hasegawa E and Emoto K (2017) Neural circuitry that evokes escape behavior in response to nociceptive stimuli in Drosophila larvae. Curr Biol 27: 2499-2504 (2017).
Yasunaga K, Tezuka A, Ishikawa N, Dairyo Y, Togashi K, Koizumi H and Emoto K (2015) Adult Drosophila sensory neurons specify dendrite boundaries independently of dendritic contacts through the Wnt5-Drl signaling pathway. Genes Dev 29: 1763-1775.
Kanamori, T., Yoshino, J., Yasunaga, K., Dairyo, Y., and Emoto, K. (2015) Local endocytosis triggers dendrite thinning and pruning in Drosophila sensory neurons. Nature Communications 6: 6515 (2015).
Kanamori, T., Kanai, M., Dairyo, Y., Yasunaga, K., Morikawa, R., and Emoto, K. (2013) Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science 340: 1475-1478.
Emoto, K., Parrish, J. Z., Jan, L., and Jan, Y. N. (2006) The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature 443: 210-213.
Emoto, K., He, Y., Ye, B., Grueber, W. B., Adler, P. N., Jan, L. Y., and Jan, Y. N. (2004) Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell 119: 245-256.Biography
I received a PhD from the Graduate School of Pharmaceutical Sciences, The University of Tokyo, working with Prof. Keizo Inoue. Postgraduate studies included work with Dr. Umeda at the Tokyo Metropolitan Institute of Medical Sciences and then with Profs. Yuh-Nung Jan & Lily Jan at the University of California San Francisco. In 2006, I became an investigator at the National Institute of Genetics and from 2010 served as Group Director, Osaka Bioscience Institute. In 2013 Professor, I joined the Graduate School of Sciences, The University of Tokyo and became the 10th head of a laboratory founded in 1877 by Prof. Edward Morse.
-
Kazuyuki Aihara, Ph.D.

Kazuyuki Aihara, Ph.D.
IRCN Executive Director / Principal Investigator
Computation
University Professor
Mathematical Modeling of Complex Systems and Applications to Neurointelligence
Research
I have been studying mathematical theory for modelling complex systems and its wide-ranging transdisciplinary applications in science and technology from the viewpoint of mathematical engineering and chaos engineering. In particular, I developed a theoretical platform composed of (1) advanced control theory of complex systems, (2) complex network theory, and (3) nonlinear data analysis and data-driven modelling. On the applications side, I am working to bridge neuroscientific and clinical studies with next-generation AI by mathematical modeling and analyses of complex brain dynamics to realize neurointelligence at IRCN.
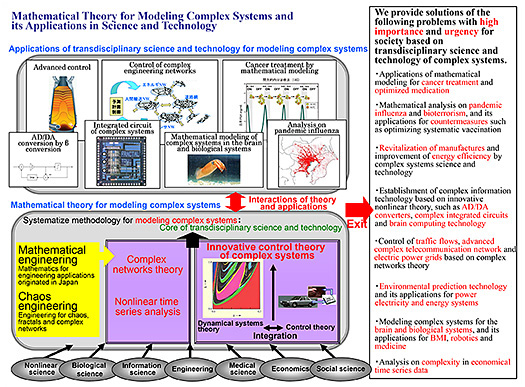
Publications
Masuda, N., Aihara, K. and MacLaren, N.G. (2024) Anticipating Regime Shifts by Mixing Early Warning Signals from Different Nodes. Nature Comm 15(1): 1086, 1-15.
Sakemi, Y., Nobukawa, S., Matsuki, T., Morie, T., and Aihara, K. (2024) Learning Reservoir Dynamics with Temporal Self-Modulation. Comm Phys 7: 29, 1-11 (2024).
Sakemi, Y., Yamamoto, K., Hosomi, T., and Aihara, K. (2023) Sparse-firing Regularization Methods for Spiking Neural Networks with Time-to-first-spike Coding. Scientific Reports 13: 22897, 1-12.
Tong, Y., Hong, R., Zhang, Z., Aihara, K., Chen, P., Liu, R., and Chen, L. (2023) Earthquake Alerting based on Spatial Geodetic Data by Spatiotemporal Information Transformation Learning. Proc Natl Acad Sci USA 120(37): e2302275120, 1-12.
Kanamaru, T., Hensch, TK., and Aihara, K. (2023) Maximal Memory Capacity Near the Edge of Chaos in Balanced Cortical E-I Networks. Neural Computation 35(8): 1430-1462.
Sakemi, Y., Morino, K., Morie, T., and Aihara, K. (2023) A Supervised Learning Algorithm for Multilayer Spiking Neural Networks Based on Temporal Coding Toward Energy-Efficient VLSI Processor Design. IEEE Trans Neural Networks and Learning Systems 34(1): 394-408.
Tanaka, G., Matsumori, T., Yoshida, H., and Aihara, K. (2022) Reservoir Computing with Diverse Timescales for Prediction of Multiscale Dynamics. Phys Rev Res 4(3): L032014, 1-7.
Aihara, K., Liu, R., Koizumi, K., Liu, X., and Chen, L. (2022) Dynamical Network Biomarkers: Theory and Applications. Gene 808: 145997, 1-10.
Inagaki, T., Inaba, K., Leleu, T., Honjo, T., Ikuta, T., Enbutsu, K., Umeki, T., Kasahara, R., Aihara, K., and Takesue, H. (2021) Collective and Synchronous Dynamics of Photonic Spiking Neurons. Nature Comm 12: 2325, 1-8.
Chen, P., Liu, R., Aihara, K., and Chen, L. (2020) Autoreservoir Computing for Multistep Ahead Prediction based on the Spatiotemporal Information Transformation. Nature Communications 11: 4568, 1-15.
Leng, S., Ma, H., Kurts, J., Lai, Y-C., Lin, W., Aihara, K., and Chen, L. (2020) Partial Cross Mapping Eliminates Indirect Causal Influences. Nature Comm 11: 2632, 1-9.
Omi, T., Ueda, N., and Aihara, K. (2019) Fully Neural Network based Model for General Temporal Point Processes. Advances in Neural Information Processing Systems 32 (NeurIPS 2019): 2120-2129.
Leleu, T., Yamamoto, Y., McMahon, P., and Aihara, K. (2019) Destabilization of Local Minima in Analog Spin Systems by Correction of Amplitude Heterogeneity. Physical Review Letters 122(4): 040607, 1-6.
Ma, H., Leng, S., Aihara, K., Lin, W., and Chen, L. (2018) Randomly Distributed Embedding Making Short-term High-dimensional Data Predictable. Proc Natl Acad Sci USA 115(43): E9994-E10002.
Schäfer, B., Beck, C., Aihara, K., Witthaut, D. and Timme, M. (2018) Non-Gaussian Power Grid Frequency Fluctuations Characterized by Lévy-stable Laws and Superstatistics. Nature Energy 3(2): 119-126.
McMahon, P.L., Marandi, A., Haribara, Y., Hamerly, R., Langrock, C., Tamate, S., Inagaki, T., Takesue, H., Utsunomiya, S., Aihara, K., Byer, R.L., Fejer, M.M., Mabuchi, H. and Yamamoto, Y. (2016) A Fully-programmable 100-spin Coherent Ising Machine with All-to-all Connections. Science 354(6312): 614-617.
Inagaki, T., Haribara, Y., Igarashi, K., Sonobe, T., Tamate, S., Honjo, T., Marandi, A., McMahon, P.L., Umeki, T., Enbutsu, K., Tadanaga, O., Takenouchi, H., Aihara, K., Kawarabayashi, K., Inoue, K., Utsunomiya, S. and Takesue, H. (2016) A Coherent Ising Machine for 2000-node Optimization Problems. Science 354(6312): 603-606.
Fujioka, E., Aihara, I., Sumiya, M., Aihara, K. and Hiryu, S (2016) Echolocating Bats Use Future-target Information for Optimal Foraging. Proc Natl Acad Sci USA 113(17): 4848-4852.
Takahashi, N., Hirata, Y., Aihara, K. and Mas, P. (2015) A Hierarchical Multi-oscillator Network Orchestrates the Arabidopsis Circadian System. Cell 163(1): 148-159
Aihara, K. (2002) Chaos Engineering and its Application to Parallel Distributed Processing with Chaotic Neural Networks. Proc IEEE 90(5): 919-930.
Biography
I received a B.E. degree in electrical engineering and Ph.D. degree in electronic engineering from the University of Tokyo (UTokyo), Tokyo, Japan, in 1977 and 1982, respectively. I led the ERATO (Exploratory Research for Advanced Technology) Aihara Complexity Modelling project for JST (Japan Science and Technology Agency) from 2003 to 2008, the FIRST Innovative Mathematical Modelling project by JSPS (Japan Society for the Promotion of Science) through the FIRST (Funding Program for World-Leading Innovative R&D Science and Technology) program from 2010 to 2014 and am now leading Aihara Moonshot project by JST and Cabinet Office of Japan . I am now University Professor、Professor Emeritus and IRCN Executive Director of the University of Tokyo, and also Special Advisor of RIKEN AIP.
-
Yukiko Gotoh, Ph.D.

IRCN Deputy Director/Principal Investigator
Development
Professor
Department of Pharmaceutical Sciences, Graduate School of Pharmaceutical Sciences, The University of TokyoNeural Development, Neural Stem/Progenitor Cells, Epigenetics, Signal Transduction
Research
A fundamental question in understanding tissue development is how resident stem cells or multipotent progenitors give rise to the various cell types in appropriate numbers and at the right locations to achieve tissue organization. Our laboratory has been studying the mechanisms and logic that underlie the regulation of neural stem/progenitor cell fate both during embryonic brain development and in the adult brain. Our current research foci include the genetic and epigenetic regulation of neural stem/progenitor cell fate and neuronal maturation, the genesis and maintenance of adult neural stem cells, and the relevance of neural stem/progenitor cell dysregulation in neurodevelopmental disorders such as autism spectrum disorders.
Publications
Furutachi, S., Miya, H., Watanabe, T., Kawai, H., Yamasaki, N., Harada, Y., Imayoshi, I., Nelson, M., Nakayama, KI., Hirabayashi, Y., and Gotoh, Y. (2015) Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat. Neurosci. 18, 657-665.
Kishi, Y., Fujii, Y., Hirabayashi, Y. and Gotoh, Y. (2012) HMGA proteins regulate global chromatin state and the neurogenic potential in neocortical precursor cells. Nat. Neurosci. 15, 1127-1133.
Hirabayashi, Y., Suzki, N., Tsuboi, M., Endo, T.A., Toyoda, T., Shinga, J., Koseki, H., Vidal, M. and Gotoh, Y. (2009) Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 63, 600-613.
Ichijo, H., Nishida, E., Irie, K., ten Dijke, P., Saitoh, M., Moriguchi, T., Takagi, M., Matsumoto, K., Miyazono, K. and Gotoh, Y. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275, 90-94.
Gotoh, Y., Nishida, E., Matsuda, S., Shiina, N., Kosako, H., Shiokawa, K., Akiyama, T., Ohta, K. & Sakai, H. (1991) In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature 349, 251-254.
Gotoh, Y., Nishida, E., Yamashita, T., Hoshi, M., Kawakami, M. & Sakai, H. (1990) MAP kinase activated by nerve growth factor and epidermal growth factor in PC12 cells. Identity with the mitogen-activated MAP kinase of fibroblastic cells. Eur. J. Biochem. 193, 661-669.Biography
As a graduate student at the University of Tokyo and later as an Assistant Professor in Kyoto University where I led a group with Eisuke Nishida, our group purified and cloned the vertebrate MAP kinase (Erk) and its activator, MAP kinase kinase (Mek). I became fascinated by the beauty of the cell fate decision where, even using the same MAPK pathway, a cell can precisely decide whether it should proliferate or differentiate. After spending a few years in Jonathan Cooper’s laboratory in Seattle and Michael Greenberg’s laboratory in Boston where I learned the basics of brain development, I started a laboratory investigating neural stem/progenitor cell fate at the University of Tokyo at the Institute of Molecular and Cellular Biosciences in 2000. I was later appointed as a Professor at the same institute in 2005 and then in the Department of Pharmaceutical Sciences of the University of Tokyo in 2014.
-
Haruo Kasai, M.D., Ph.D.

Principal Investigator
Technology
Project Professor
Neural Circuit and Synaptic Mechanisms of Memory and Cognitive Function
Research
Neuronal circuits formed by synapses in the brain enable learning, memory, perception, and emotion, and their impairment results in various mental disorders. We extensively utilize two-photon microscopy to observe cellular and molecular events deep within the brain, enabling optical recording, manipulation and electrophysiology of synapses and neuronal circuits. By developing two-photon uncaging, we demonstrated rapid enlargement of dendritic spines during potentiation in the hippocampus, neocortex, and basal ganglia, underpinning long-term potentiation (LTP), dopamine modulation of single dendritic spines, intrinsic synaptic dynamics and rapid mechanical actions of spine enlargement on presynaptic terminal (mechanical synaptic transmission) preceding LTP. We also invented Synaptic Chemogenetics (SYNCit) to manipulate spine synapses in broad cortical areas, revealing dendritic spine control over sleep homeostasis, cortical wakefulness, and rapid cognitive functions. Our advancements in molecular labeling of active spines together with SYNCit approaches, visualizing the cellular basis of memory and cognition, new treatments for mental disorders, and novel concepts for artificial intelligence.
Publications
Sawada,T., Iino, Y., Yoshida, K., Okazaki, H., Nomura, S., Shimizu, C., Arima, T., Juichi, T., Zhou, S., Kurabayashi, N., Sakurai, T., Yagishita, S., Yanagisawa, M., Toyoizumi, T., Kasai, H.*, Shi, S.* (2024) Prefrontal synaptic regulation of homeostatic sleep pressure revealed through synaptic chemogenetics. Science, 385, 1459-1465.
Ucar, H., Morimoto, Y., Watanabe, S., Noguchi, J., Iino, Y., Yagishita, S., Takahashi, N. and Kasai, H.* Mechanical actions of dendritic-spine enlargement on presynaptic exocytosis. Nature 600; 686-689, 2021.
Iino, Y., Sawada, T., Yamaguchi, Tajiri, M., K., Ishii, S., Kasai, H.* and Yagishita, S.* Dopamine D2 receptors in discrimination learning and spine enlargement. Nature, 579; 555-560, 2020.
Hayashi-Takagi, A., Yagishita, S., Nakamura, M. Shirai, F., Wu, Y., Loshbaugh, A.L., Kuhlman, B., Hahn, K.M. and Kasai, H.* Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525; 333-338, 2015.
Yagishita, S., Hayashi-Takagi, A., Ellis-Davies, G.C.R., Urakubo, H., Ishii, S. and Kasai, H.* A critical time window for dopamine action on the structural plasticity of dendritic spines. Science 345; 1616-1620, 2014.
Tanaka, J., Horiike, Y., Matsuzaki, M., Miyazaki, T., Ellis-Davies, G.C.R. and Kasai, H.* Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 319; 1683-1687, 2008.
Matsuzaki, M., Honkura, N., Ellis-Davies, G.C.R. and Kasai, H.* Structural basis of long-term potentiation in single dendritic spines. Nature 429; 761-766, 2004.
Biography
Haruo Kasai graduated from the University of Tokyo School of Medicine, training in Masao Ito's laboratory, and became a Humboldt Fellow at the Max-Planck Institute, Germany, under Erwin Neher. Upon returning to Japan, he served as Associate Professor of Physiology at the University of Tokyo and later as Professor at the National Institute of Physiological Sciences, pioneering two-photon uncaging of caged glutamate and discovered enlargement of dendritic spine. Returning to the Medical School of the University of Tokyo as Professor and becoming a PI at IRCN, he developed various new methods and discovered fast mechanical actions of spine enlargement on synaptic transmission and its roles in cognitive function.
-
Kiyoto Kasai, M.D., Ph.D.

Principal Investigator
Human/Clinical
Professor
Department of Neuropsychiatry, Graduate School of Medicine, The University of TokyoNeuroimaging and Clinical Neurophysiology in Psychiatric Disorders
Research
My research focuses on the biological mechanisms underlying psychosis onset and the development of effective early intervention strategies. Our group’s expertise includes a wide range of neuroimaging techniques, such as magnetic resonance imaging (MRI), MR spectroscopy, and EEG. We extended our studies to adolescent brain neuroscience via the launch of the Tokyo TEEN Cohort in 2012, which is the first large-scale population-based cohort study of adolescents in Asia. I am also one of the principal investigators of Japan’s national brain project called Brain/MINDS 2.0, organizing an all-Japan multi-site MRI research framework in psychiatric disorders.
Publications
Nagaoka D et al: Identify adolescents’ help-seeking intention on suicide through self- and caregiver's assessments of psychobehavioral problems: deep clustering of the Tokyo TEEN Cohort study. Lancet Regional Health Western Pacific. Volume 43, February 2024.
ENIGMA Clinical High Risk for Psychosis Working Group: Using brain structural neuroimaging measures to predict psychosis onset for individuals at clinical high-risk. Mol Psychiatry. 2024 Feb 9.
Okada N et al: Longitudinal trajectories of anterior cingulate glutamate and subclinical psychotic experiences in early adolescence: The impact of bullying victimization. Mol Psychiatry. 05 January 2024.
Okada N et al: Subcortical volumetric alterations in four major psychiatric disorders: a mega-analysis study of 5604 subjects and a volumetric data-driven approach for classification. Mol Psychiatry. 2023 Aug 4.
Kasai K, et al: Strengthening community mental health services in Japan. Lancet Psychiatry 4: 268-270, 2017. (Viewpoint)
Okano H, et al: Brain/MINDS: A Japanese national brain project for marmoset neuroscience. Neuron 92: 582-590, 2016. (Viewpoint)
Okada N, et al: Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol Psychiatry 21: 1460-1466, 2016.
Kasai K, Shenton ME, Salisbury DF, et al: Progressive decrease of left Heschl gyrus & planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 60(8):766-775, 2003.
Biography
I earned an M.D. in 1995 and Ph.D. in Psychiatry in 2004, both from the University of Tokyo. I gained clinical experience at the University of Tokyo Hospital and National Center of Neurology and Psychiatry, before an appointment as visiting instructor at Harvard Medical School, USA, from 2000 to 2002. I returned to the University of Tokyo Hospital, Department of Neuropsychiatry in 2002 as Instructor, and was appointed Lecturer in 2003 and Professor and Chair of the Department of Neuropsychiatry in 2008. I was a recipient of the Distinguished Investigator Award from the Japanese Society of Biological Psychiatry in 2003, and the Young Investigator Award from the Japan Neuroscience Society in 2008.
-
Kenichi Ohki, M.D., Ph.D.

IRCN Deputy Director/Principal Investigator
Development
Professor
Department of Physiology, Graduate School of Medicine, The University of TokyoFunctional organization and development of the visual system
Research
I am interested in visual neuroscience and functional brain mapping. In 2005, I developed a method of single-cell resolution functional mapping with two-photon calcium imaging, and was able to measure the orientation selectivity of hundreds of neurons in visual cortex revealing the functional architecture of visual cortex between rodents and higher mammals (Ohki et al., 2005, Nature). In 2006, using this method, I solved a long-standing problem in visual neuroscience - the micro-architecture of pinwheel centers at the single-cell level (Ohki et al., 2006). In 2012, I found that neurons derived from the same neural stem cells tend to acquire similar orientation selectivity in the adult mouse visual cortex (Ohtsuki et al., 2012) and later that neuronal activity is not required for the initial formation of orientation selectivity, but required for the later reorganization (Hagihara et al., 2015). Our lab continue to study the interplay between innate circuits determined by the developmental programs, and neuronal activity in determining the functions of neurons in the cerebral cortex.
Publications
Matsui T, Murakami T, Ohki K. Transient neuronal coactivations embedded in globally propagating waves underlie resting-state functional connectivity. Proc Natl Acad Sci U S A. 113:6556-61 (2016).
Kondo S, Ohki K. Laminar differences in the orientation selectivity of geniculate afferents in mouse primary visual cortex. Nat Neurosci.,19: 316-9 (2016).
Hagihara KM, Murakami T, Yoshida T, Tagawa Y, Ohki K. Neuronal activity is not required for the initial formation and maturation of visual selectivity. Nat Neurosci.,18: 1780-8 (2015).
Kawashima T, Kitamura K, Suzuki K, Nonaka M, Kamijo S, Takemoto-Kimura S, Kano M, Okuno H, Ohki K, Bito H. Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nat Methods. 10: 889-895 (2013).
Ohtsuki G, Nishiyama M, Yoshida T, Murakami T, Histed MH, Lois C, Ohki K Similarity of visual selectivity among clonally related neurons in visual cortex. Neuron. 75: 65-72 (2012).
T. Mrsic-Flogel, S. B. Hofer, K. Ohki, R. C. Reid, T. Bonhoeffer, M. Hubener. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. Vol. 54:961-72 (2007).
Ohki K, Chung S, Kara P, Hubener M, Bonhoeffer T, Reid RC. Highly ordered arrangement of single neurons in orientation pinwheels. Nature 442: 925-928 (2006).
Ohki K, Chung S, Ch'ng YH, Kara P, Reid. RC Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433: 597-603 (2005).
Ohbayashi M, Ohki K, Miyashita Y. Conversion of working memory to motor sequence in the monkey premotor cortex. Science 301: 233-6 (2003).
H. Kikyo, K. Ohki, Y. Miyashita. Neural correlates for feeling-of-knowing: an fMRI parametric analysis. Neuron 36: 177-86 (2002).
Biography
1990-1996 Medical student, Faculty of Medicine, The University of Tokyo (MD)
1996-2000 PhD student, Department of Physiology, The University of Tokyo (PhD)
1996-1996 Visiting scholar, Department of Brain and Cognitive Sciences, MIT
2000-2002 Assistant Professor, Department of Physiology, The University of Tokyo
2002-2008 Research Fellow, Department of Neurobiology, Harvard Medical School
2008-2010 Instructor, Department of Neurobiology, Harvard Medical School
2010-2016 Professor, Department of Molecular Physiology, Kyushu University
2016-today Professor, Department of Physiology, The University of Tokyo -
Yasushi Okada, Ph.D.
Principal Investigator
Technology
Professor
Department of Cell Biology, Graduate School of Medicine, The University of TokyoTechnology Development for In Vivo Imaging of Cell Dynamics
Research
Our laboratory aims to deepen our understanding of the fundamental question, "What is life?" While recent advancements in molecular and structural biology have significantly advanced our understanding of life phenomena, this essential question remains unanswered.
The hallmark of our approach is the direct observation of life phenomena at the molecular level within living cells, rather than relying on imaginary illustrations or static models. To realize this vision, we are focusing on three key areas of technological development:
1. High-resolution live-cell imaging techniques: Development of cutting-edge microscopy technologies to directly observe molecules functioning within living cells.
2. Cell state visualization probes: Design and synthesis of innovative molecular probes to visualize dynamic changes in cellular states.
3. Image analysis methods: Development of mathematical and computational methods to extract valuable information from microscopic images.
By integrating these technologies and applying them to the observation of neurons, we aim to contribute to the elucidation of brain function and mechanisms of neurological disorders.
Our research is positioned at the intersection of biology, physics, chemistry, and information science. Through this interdisciplinary approach, we strive to open new frontiers in life sciences.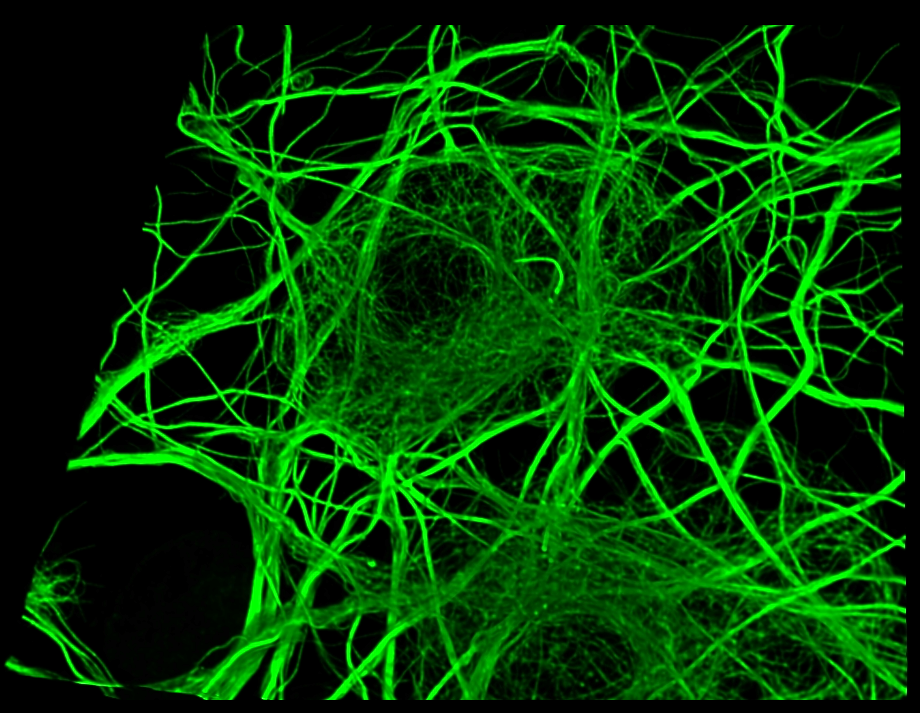
Publications
Kondratiev AY, Inutsuka Y, Okada Y. Continuous cell cycle representation using ordinal regression and siamese network from quantitative phase images. ISBI2024, 2024.
Wu Q, Taki M*, Tanaka Y, Kesherwani M, Phung QM, Enoki S, Okada Y*, Tama F*, Yamaguchi S*. Stereochemistry-Dependent Labeling of Organelles with a Near-Infrared-Emissive Phosphorus-Bridged Rhodamine Dye in Live-Cell Imaging. Angewandte Chemie International Edition, 63: e202400711, 2024.
Ando R, Shimozono S, Ago H, Takagi M, Sugiyama M, Kurokawa H, Hirano M, Niino Y, Ueno G, Ishidate F, Fujiwara T, Okada Y, Yamamoto M, Miyawaki A. StayGold variants for molecular fusion and membrane-targeting applications. Nat Methods. 2023.
Ishikawa S, Iwanaga Y, Ueyama T, Li X, Hojo H, Fujinaga I, Katashima T, Saito T, Okada Y, Chung U, Sakumichi N, Sakai T. Percolation-induced gel–gel phase separation in a dilute polymer network. Nat. Mater. 22: 1564–1570, 2023.
Okamoto K, Fujita H, Okada Y, Shinkai S, Onami S, Abe K, Fujimoto K, Sasaki K, Shioi G, Watanabe TM. Single-molecule tracking of Nanog and Oct4 in living mouse embryonic stem cells uncovers a feedback mechanism of pluripotency maintenance. EMBO J. 42: e112305, 2023.
Fukuda T, Furukawa K, Maruyama T, Yamashita SI, Noshiro D, Song C, Ogasawara Y, Okuyama K, Alam JM, Hayatsu M, Saigusa T, Inoue K, Ikeda K, Takai A, Chen L, Lahiri V, Okada Y, Shibata S, Murata K, Klionsky DJ, Noda NN, Kanki T. The mitochondrial intermembrane space protein mitofissin drives mitochondrial fission required for mitophagy. Mol Cell. 83: 2045-2058.e9, 2023.
Nozaki T, Shinkai S, Ide S, Higashi K, Tamura S, Shimazoe MA, Nakagawa M, Suzuki Y, Okada Y, Sasai M, Onami S, Kurokawa K, Iida S, Maeshima K. Condensed but liquid-like domain organization of active chromatin regions in living human cells. Science Advances. 9: eadf1488, 2023.
Katoh TA, Omori T, Mizuno K, Sai X, Minegishi K, Ikawa Y, Nishimura H, Itabashi T, Kajikawa E, Hiver S, Iwane AH, Ishikawa T, Okada Y, Nishizaka T, Hamada H. Immotile cilia mechanically sense the direction of fluid flow for left-right determination. Science. 379: 66-71, 2023.
Nagao Y, Sakamoto M, Chinen T, Okada Y, Takao D. Robust classification of cell cycle phase and biological feature extraction by image-based deep-learning. Mol Biol Cell 31: 1346-1354, 2020.
Fujioka Y, Alam JM, Noshiro D, Mouri K, Ando T, Okada Y, May AI, Knorr RL, Suzuki K, Ohsumi Y, Noda NN. Phase separation organizes the site of autophagosome formation. Nature 578: 301-305, 2020.
Biography
Graduated from the University of Tokyo, Faculty of Medicine in 1993. Medical Doctor (M.D.) and Ph.D. After serving as a JSPS Research Fellow and Assistant Professor at the same university's graduate school, became a Team Leader at RIKEN Center for Biosystems Dynamics Research (formerly known as RIKEN Quantitative Biology Center until reorganization in 2018) in 2011. Since 2016, Professor at the Department of Physics, Graduate School of Science, The University of Tokyo (concurrent position with RIKEN). Since 2020, Professor at the Department of Molecular and Cellular Biology, Graduate School of Medicine, The University of Tokyo (concurrent positions with RIKEN and Graduate School of Science).
Specializes in cell biology and biophysics. Conducts research utilizing various imaging technologies, with a focus on optical microscopy (single-molecule imaging, super-resolution microscopy techniques). -
Shoji Takeuchi, Ph.D.

Principal Investigator
Technology
Professor
Department of Mechano-Informatics, Graduate School of Information Science and Technology, The University of TokyoBiohybrid systems, Micro electro mechanical systems,
Microfluidics, Tissue Engineering, Artificial cell membraneResearch
Our group focuses on the design and fabrication of bio-hybrid systems that combine bio functional materials with micro/nano devices. The development of microfabrication technologies has realized ultrasmall sensors and actuators at micro and nanometer scale, although the real biological systems are often more sensitive, efficient and functional than the micromachines. One idea to solve this problem is to create hybrid systems by fusing the mechanical components with biomaterials such as biological cells and molecular machines. For example, we have succeeded in the fabrication of microelectrodes which have small microfluidic channels using a flexible polymer to stimulate neural cells chemically and detect neural signals electrically. Biohybrid neural probes which can connect artificial systems and brain will contribute to a better understanding of our brain.
Publications
Yuya Morimoto, Hiroaki Onoe and Shoji Takeuchi: Biohybrid robot powered by an antagonistic pair of skeletal muscle tissues. Science Robotics, vol. 3, eaat4440, 2018
Koki Kamiya, Ryuji Kawano, Toshihisa Osaki, Kazunari Akiyoshi, and Shoji Takeuchi:Cell-sized asymmetric lipid vesicles facilitate the investigation of asymmetric membranes, Nature Chemistry, vol. 8, pp. 881-889, 2016
Shigenori Miura, Koji Sato, Midori Kato-Negishi, Tetsuhiko Teshima and Shoji Takeuchi: Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6, Nature Communications, vol. 6, 8871, 2015
Won Chul Lee, Kwanpyo Kim, Jungwon Park, Jahyun Koo, Hu Young Jeong, Hoonkyung Lee, David Weitz, Alex Zettl, and Shoji Takeuchi: Graphene-templated directional growth of an inorganic nanowire, Nature Nanotechnology, vol. 10, pp. 423-428, 2015
Hiroaki Onoe, Teru Okitsu, Akane Itou, Midori Kato-Negishi, Riho Gojo, Daisuke Kiriya, Koji Sato, Shigenori Mirua, Shintaroh Iwanaga, Kaori Kuribayashi-Shigetomi, Yukiko Matsunaga, Yuto Shimoyama, and Shoji Takeuchi: Metre-long Cell-laden Microfibres Exhibit Tissue Morphologies and Functions, Nature Materials, vol.12, pp. 584-590, 2013
Yun Jung Heo, Hideaki Shibata, Teru Okitsu, Tetsuro Kawanishi, and Shoji Takeuchi: Long-term in vivo glucose monitoring using fluorescent hydrogel fibers, Proc. Natl. Acad. Sci. USA, vol. 108(33), pp. 13399-13403, 2011
Hideaki Shibata, Yun Jung Heo, Teru Okitsu, Yukiko Matsunaga, Tetsuro Kawanishi, and Shoji Takeuchi: Injectable hydrogel microbeads for fluorescence-based continuous glucose monitoring,Parylene-coating in PDMS microfluidic channels prevents the absorption of fluorescent dyes, Proc. Natl. Acad. Sci. USA,vol. 107, no. 42, pp. 17894-17898, 2010
N. Misawa, H. Mitsuno, R. Kanzaki, and S. Takeuchi: A Highly Sensitive and Selective Odorant Sensor using Living Cells Expressing Insect Olfactory Receptors, Proc. Natl. Acad. Sci. USA , vol. 107(35), pp. 15340-15344, 2010
Wei-Heong TAN and Shoji TAKEUCHI: A Trap-and-Release Integrated Microfluidic System for Dynamic Microarray Applications, Proc. Natl. Acad. Sci. USA, vol. 104, no. 4, pp. 1146-1151, 2007Biography
I received the B.E., M.E., and Dr. Eng. degrees in mechanical engineering from the University of Tokyo, Tokyo, Japan in 1995, 1997, and 2000, respectively. Currently, I am a Professor and Director of the Center for International Research on Integrative Biomedical Systems (CIBiS), Institute of Industrial Science (IIS) at the University of Tokyo. My team and I have authored more than 160 peer-reviewed publications and filed over 70 patents, and I have been recognized with honors, including the MEXT Young Scientists' Prize in 2008, the JSPS prize in 2010, and the ACS Analytical Chemistry Young Innovator Awards in 2015. My current research interests include 3D tissue fabrication, implantable devices, artificial cells/lipid bilayer systems, and biohybrid MEMS.


