Electroencephalography (EEG), which records electrical discharges in the brain, is a well-established technique for measuring brain activity. But current EEG electrode arrays, even placed directly on the brain, cannot distinguish the activity of different types of brain cells, instead averaging signals from a general area. Nor is it possible to easily compare EEG data with brain imaging data.
A collaboration between neuroscientist Michela Fagiolini, PhD at Boston Children’s Hospital and engineer Hui Fang, PhD at Northeastern University has led to a highly miniaturized, see-through EEG device. It promises to be much more useful for understanding the brain’s workings.
Tiny and transparent
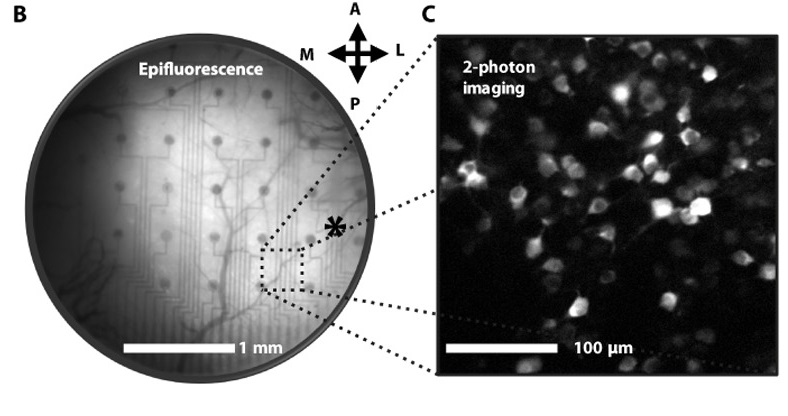
The new EEG microarray, described today in Science Advances, combines two key advantages. First, the electrodes are placed very close together on the surface of the cortex — a few micrometers apart, as opposed to millimeters currently. This enables researchers to collect very fine-grained information, down to the level of single neurons.
Second, the team applied nanotechnologies to make the electrodes transparent. This allows light to pass through the electrode array so that the brain can be imaged at the same time.
In experiments, Fagiolini and her team placed this transparent microarray on the visual cortex of live, awake mice. They were able to capture the electrical outputs of individual neurons as they responded to visual stimuli, while simultaneously obtaining high-resolution optical images.
“This allows us to do experiments that were not possible before,” says Fagiolini, who studies alterations of visual perception in Rett syndrome in Boston Children’s F.M. Kirby Neurobiology Center. “In Rett syndrome, for example, we can know which specific groups of neurons are generating the abnormal EEG signals we’ve been observing. Or we can see how particular cell populations are impacted by abnormal electrical signaling.”
The transparent nature of the electrodes also enables researchers to do optogenetics experiments (genetically altering cells using light) in conjunction with EEG. “We can then ask, if I perturb these cells, what happens to the EEG?” says Fagiolini.
Building a better microelectrode array
Fang, co-senior author on the paper with Fagiolini, explains that transparent microelectrodes have been developed in the past using different materials such as graphene, but suffer from poor performance. Fang’s team went back to existing electrode materials and turned to nanotechnology to fabricate a fine, two-layered mesh.
“What’s remarkable is that by simple nanomeshing, we show that conventional electrode materials can be made transparent, while not compromising electrode performance,” says Fang, whose lab is developing novel neurotechnologies in Northeastern’s Electrical and Computer Engineering Department.
Another helpful feature is that the microelectrode arrays are soft, flexible and more biocompatible. This allows them to be implanted more safely on the brain, or a section of the brain, and remain for longer periods. Unlike most current electrodes, which are rigid, they conform to the brain’s curvature — a helpful feature for application to humans, whose brains are highly convoluted.
Advancing neuroscience with transparent microelectrodes
Fagiolini envisions using the technology to study a variety of neurologic conditions, including traumatic brain injury and spinal cord injury. Interest is spreading among neuroscientists at Boston Children’s. Fagiolini plans to work with hospital colleagues to develop computational algorithms to better interpret the paired EEG and imaging data.
The team has filed a patent and plans to further refine the electrode microarray, make the system wireless and move to testing in primates and eventually humans. Patients undergoing neurosurgery for conditions such as epilepsy could be among the first test subjects, since they already have EEG arrays implanted to help identify a safe path for surgery.
A much longer-range goal is to stimulate neurons through the electrodes to adjust their activity, rather than simply record their signals. Such stimulation could potentially have therapeutic applications.
Yi Qiang and Kyung Jin Seo of Northeastern University and Pietro Artoni of Boston Children’s Hospital are co-first authors on the paper. The study was supported by Northeastern University, the NIH National Institute of Neurological Disorders and Stroke (R01NS095959), Rettsyndrome.org, Rett Syndrome Research Trust, Simon Foundation, Human Frontier Science Program–Cross-Disciplinary Fellowships, Endowment for the Chan Soon-Shiong Bionic Engineering Research Center, and University of California, Los Angeles. See the paper for a full list of authors and acknowledgements.
See News@Northeastern for more on this story, and read more about research at the FM Kirby Neurobiology Center.