Do we know how long we are supposed to sleep each night? How about our children or even loved pets? Sleep researchers have long known that sleep duration (the length of total sleep) is strongly influenced by genetic background. So, the time we spend sleeping is usually more similar to our siblings than our spouses. However, how the nightly sleep duration is regulated and the intracellular mechanisms are yet undefined.
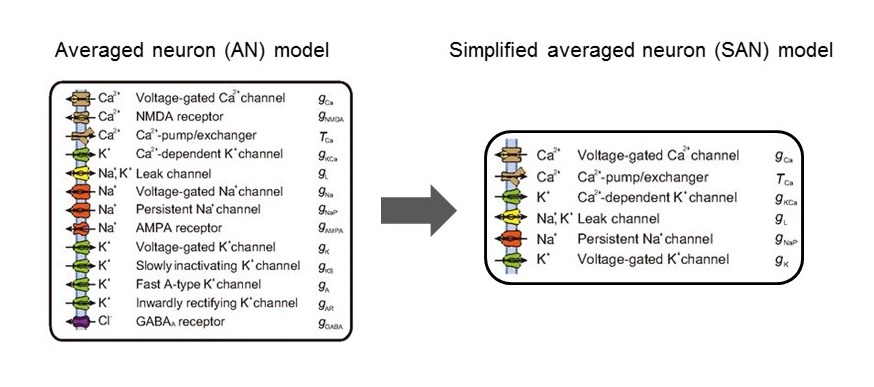
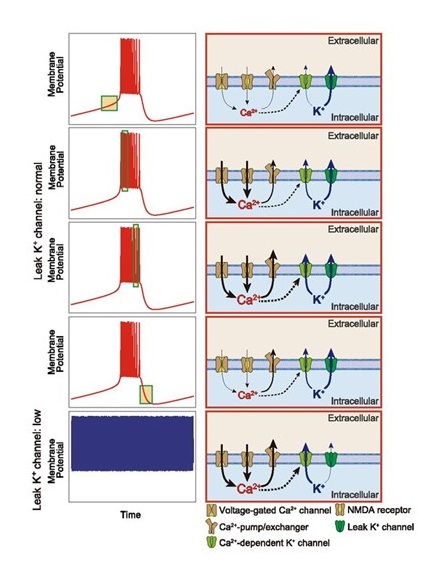
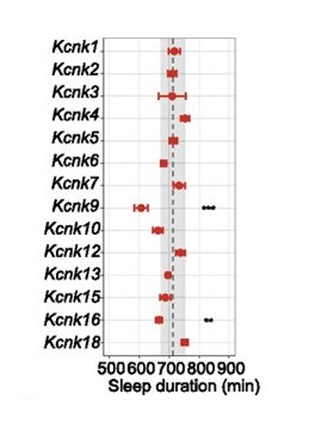
Professor Hiroki Ueda of the International Research Center for Neurointelligence (IRCN), Graduate School of Medicine, the University of Tokyo, and RIKEN Center for Biosystems Dynamics Research, approached this question by focusing on describing the firing patterns of cortical neurons during non-rapid eye movement (NREM) sleep with a new computational model. The firing pattern during NREM sleep is represented as a canonical slow-wave sleep (SWS, equal to deep NREM sleep) firing pattern. Compared with patterns during wakefulness or REM sleep, the SWS firing pattern displays depolarized “up” states and hyperpolarized “down” states. Previously, Ueda’s team proposed a computational model to understand the mechanism of the SWS firing pattern by integrating a Ca2+ dependent hyperpolarized pathway (Tatsuki et al., Neuron 90:70-85, 2016). In the current study, they further explored the mechanism of the down state occurring during NREM sleep, adapting the original model into an simplified one in which the main components are reduced from 13 to 6 (five channels and a pump, Figure 1). Based on this mathematical analysis of the transition from the up to down states in the SWS firing pattern, the new model revealed the involvement of leak K+ channels as an electrophysiological characteristic of SWS (Figure 2). To examine this hypothesis, the research team generated 14 different knockout (KO) mice for various channel genes by the CRISPR-Cas9 system and conducted sleep phenotyping using a respiration-based, non-invasive Snappy Sleep Stager (Figure 3). When validated by EEG/EMG polysomnography, Kcnk9 KO mice showed decreased total sleep time reflecting a shortened duration of NREM but not REM sleep.
These results demonstrate the significance of leak K+ channels in regulating NREM sleep duration. A novel aspect of the study is that the findings were driven by the computational analytical simulation, and through this calculated hypothesis the researchers verified the importance of a specific gene’s function in the sleep-wake cycle. With this result, Ueda’s team successfully unveiled another possible molecular mechanism that generates rhythmic cortical firing patterns during NREM sleep that in turn determines nightly sleep duration. This computational tool has promise to solve further mysteries of sleep regulation and in the future waking-state intelligence states.
Correspondent: Mayumi Kimura, Ph.D., IRCN Science Writing Core
Reference: Yoshida K, Shi S, Ukai-Tadenuma M, Fujishima H, Ohno R, and Ueda HR (2018) Leak potassium channels regulate sleep duration. Proc. Natl. Acad. Sci. USA 115: E9459-E9468. Published online: September 17, 2018. DOI: 10.1073/pnas.1806486115
Graphic: see below
Media Contact: The author is available for interviews in English.
Professor Hiroki Ueda, M.D., Ph.D.
Department of Systems Pharmacology, Graduate School of Medicine
International Research Center for Neurointelligence
The University of Tokyo
Mayuki Satake
Public Relations
International Research Center for Neurointelligence
The University of Tokyo
satake.mayuki@mail.u-tokyo.ac.jp


