In Brief:
Early-life seizures are often associated with intellectual disability and/or autism. Sun et al. show that seizures prematurely unsilence synapses to disrupt tonotopic plasticity in auditory cortex, revealing a mechanism for the relationship between seizures and later cognitive impairment.
Highlights:
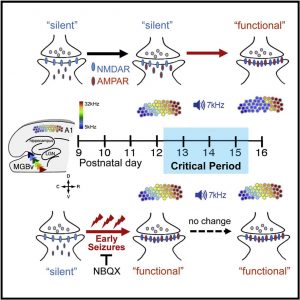
- Early-life seizures disrupt a critical period for tonotopic map plasticity in A1
- Maturational decrease in NMDA-only silent synapses characterizes this CP
- Seizures accelerate synapse unsilencing by AMPA receptor insertion
- An AMPAR antagonist prevents synapse unsilencing and rescues CP plasticity
Graphical Abstract:
Summary:
Heightened neural excitability in infancy and child-hood results in increased susceptibility to seizures. Such early-life seizures are associated with language deficits and autism that can result from aberrant development of the auditory cortex. Here, we show that early-life seizures disrupt a critical period (CP) for tonotopic map plasticity in primary auditory cor-tex (A1). We show that this CP is characterized by a prevalence of ‘‘silent,’’ NMDA-receptor (NMDAR)-only, glutamate receptor synapses in auditory cortex that become ‘‘unsilenced’’ due to activity-dependent AMPA receptor (AMPAR) insertion. Induction of sei-zures prior to this CP occludes tonotopic map plasticity by prematurely unsilencing NMDAR-only synapses. Further, brief treatment with the AMPAR antagonist NBQX following seizures, prior to the CP, prevents synapse unsilencing and permits sub-sequent A1 plasticity. These findings reveal that early-life seizures modify CP regulators and suggest that therapeutic targets for early post-seizure treat-ment can rescue CP plasticity.
Journal Articles:
Cell Reports 23, May 29, 2018
https://www.cell.com/cell-reports/fulltext/S2211-1247(18)30706-X



