Disclaimer: machine translated by DeepL which may contain errors.
Tuning of peripheral pain sensation according to the nutritional status of individuals.
Mami DOUE (Doctoral Student at the time of research / JSPS Research Fellow, The University of Tokyo)
Kenichi Ishii (Assistant Professor, Department of Biological Sciences)
Jiro Yoshino (Postdoctoral Fellow, Department of Biological Sciences)
Masato Tsuji (Assistant Professor, Department of Biological Sciences)
Kazuo Emoto (Professor, Department of Biological Sciences / Deputy Director and Senior Staff, International Research Center for Neurointelligence (WPI-IRCN))
Key Points of the Presentation
- In Drosophila larvae, the pain response is suppressed when sugar is fed after a period of temporary hunger.
- We found a group of neurons "SDGs" in the fly brain that are responsible for the inhibition of pain sensation by sugar feeding, and clarified how they are activated by the insulin pathway to modulate peripheral pain sensation.
- This research is expected to elucidate the operating principle of peripheral pain tuning in response to the nutritional status of individuals and to bring new concepts for the development of pain treatment methods.
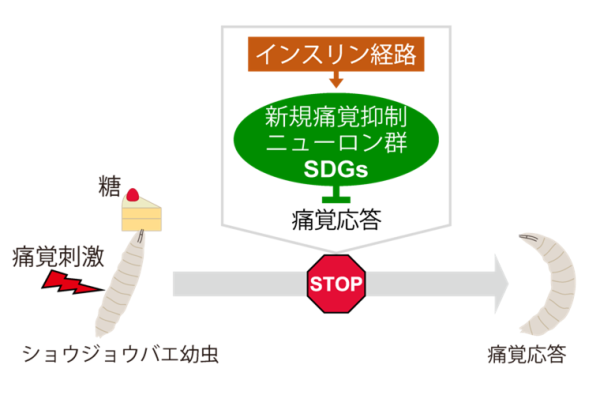
Brain Mechanism of Inhibition of Pain Response by Sugar Ingestion
Summary of Presentation
While animals are exposed to danger while obtaining food, they need to appropriately switch priorities between "escape" and "feeding" depending on the situation in order to increase their chances of survival and reproduction. In such behavioral choices, the cranial nervous system plays an important role in regulating the pain response depending on the nutritional status of the individual, but this mechanism has not been fully understood until now.
In this study, a group led by Graduate Student Mami Doue (Nakamizo), Assistant Professor Kenichi Ishii, Postdoctoral Researcher Jiro Yoshino, Assistant Professor Masato Tsuji, and Professor Kazuo Emoto (Deputy Director and Senior Staff, WPI-IRCN) of the Department of Biological Sciences, Graduate School of Science, The University of Tokyo, discovered a novel group of neurons that suppress the pain response by transmitting information from the center to the periphery in Drosophila larvae and named them SDGs. Furthermore, they investigated the physiological relationship between the nutritional status of the individual and the neural activity of SDGs, and found that sugar feeding activates SDGs via the insulin pathway, leading to analgesic effects. This study is the first example of linking the mechanisms in the brain that flexibly modulate pain responses in response to the physiological state of an individual at the molecular, neural circuit, and behavioral levels.
Presentation Details
The pain response in animals dynamically changes depending on internal conditions such as nutrition. This suggests that there is a neural mechanism that monitors physiological conditions in the body and modulates the pain response in the periphery. However, due to the complexity of the neural circuits in mammals, it has been difficult to capture the substance of the nervous system that regulates the pain response in response to nutritional conditions. In this study, we used Drosophila, which has a simpler neural circuit than mammals, to search for a group of neurons that modulate peripheral pain sensation via the central nervous system, and used them as a starting point to elucidate the nutrition-dependent control mechanism of pain sensation.
The pain response is triggered by nociceptive neurons (Note 1) that sense various stimuli. The research group focused on a group of neurons with a characteristic morphology in the fly larval brain that extend from the central nervous system to the vicinity of nociceptive neurons (Figure 1A). They then genetically inhibited the activity of each of these neurons and examined the pain responsiveness (Note 2) of the larvae (Figure 1B). As a result, we succeeded in finding a group of six pain-suppressing neurons that had never been analyzed before and named them SDGs (Note 3). Detailed analysis of brain tissue revealed that SDGs "put the brakes" on the pain response by releasing GABA (Note 4) and transmitting inhibitory signals to the presynaptic terminals of nociceptive neurons (Note 5).
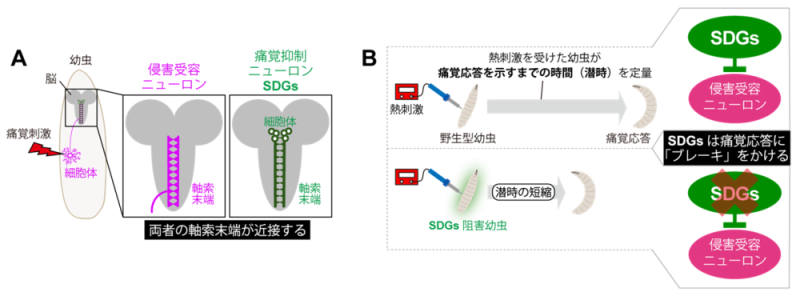
Figure 1: Morphology and function of novel pain suppressor neuron groups (SDGs).
What are the roles of the discovered pain suppressor neurons? Taking a cue from the mammalian phenomenon that "eating sugar relieves pain," the research group turned their attention to the relationship between nutrition and pain. First, when larvae were fed sugar while in a state of temporary hunger, their pain-responsiveness weakened, indicating pain modulation in response to nutritional status in flies (Fig. 2). On the other hand, larvae in which the neural activity of SDGs was suppressed showed no pain suppression by sugar feeding, indicating that SDGs are required for this phenomenon (Figure 2). Next, focusing on the insulin pathway involved in sugar metabolism, we artificially manipulated the function of insulin receptors in SDGs, and found that the neural activity of SDGs and the pain responsiveness of larvae were altered after sugar feeding. These results suggest a model in which the insulin-like peptide (Note 6) secreted by sugar feeding acts directly on SDGs, leading to reduced pain responsiveness (Figure 2).
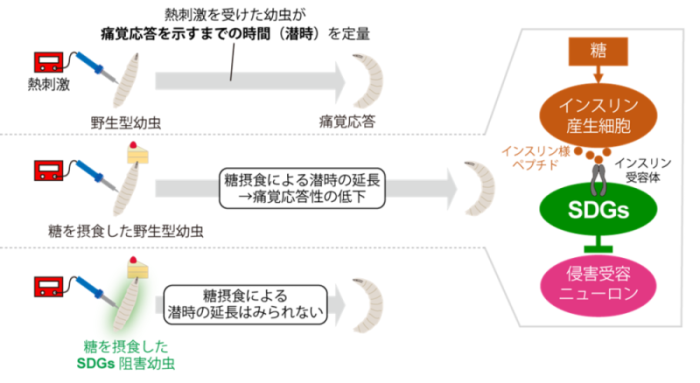
Figure 2: Regulatory mechanism of nutritional status-dependent pain response via SDGs.
Since the SDGs found in this study are located in brain regions that integrate diverse sensory information, they may be responsible for pain regulation in response not only to the internal state of nutrition, but also to the external environment surrounding the individual. In addition, since the function of fly SDGs is similar to a part of the human pain control system, this research is expected to lead to the development of new pain treatments and analgesics.
Publication Information
〈Journal Title〉Nature Communications
〈Title of paper〉Descending GABAergic pathway links brain sugar-sensing to peripheral nociceptive gating in Drosophila
〈Author(s)〉Mami Nakamizo-Dojo, Kenichi Ishii, Jiro Yoshino, Masato Tsuji, and Kazuo Emoto* (author)
〈DOI Number〉 10.1038/s41467-023-42202-9
Research Grant
This research is supported by the Grant-in-Aid for Scientific Research "Dynamic Control of Brain Functions by Scrap and Build (Project No. 16H06456)," "Molecular Cellular Basis for Organization, Maintenance and Management of Neural Circuits (Project No. 16H02504)," "AMED-CREST: Tissue Developmental Control of Pain Sensitivity AMED-CREST "Comprehensive Understanding of Tissue Developmental Control Mechanisms of Pain Sensitivity and Creation of New Research Platforms (Project No. JP21gm1310010)," MEXT Distinguished Researcher "Neural Basis of Social Discomfort Emotions that Regulate Hierarchical Relationships among Individual Animals (Project Leader: Kenichi Ishii)," Grant-in-Aid for Scientific Research "Neural Basis of Aggression Control Genes that Regulate Hierarchical Relationships among Individual Animals Ken-ichi Ishii, "Pain and loneliness: Neural basis of sociality formation through peripheral pain tuning (JP22gm6510011)", AMED-PRIME, "Neural basis of escape behavior timeframe", Grant-in-Aid for Scientific Research on Innovative Areas, "Neural basis of escape behavior timeframe". Elucidation of the Neural Basis for the Time Frame of Behavior (Assignment no. 20J22063), PI: Mami Dojo (Nakamizo)," and others.
Terminology
Note1 Nociceptive neuron
Peripheral sensory neurons that detect noxious stimuli that may cause physical damage to the animal and are the first to act in eliciting a pain response.
Note 2 Pain response
When fly larvae are exposed to nociceptive stimuli such as stinging by parasitic bees or high heat, they quickly roll and escape like a "corkscrew". In this study, we analyzed the behavior of the larvae by considering this rolling motion as a pain response of the larvae, and evaluated pain responsiveness as an index of the time from the time of receiving the stimulus to the start of rolling.
Note 3 SDGs
The name of a group of neurons identified in this study, which stands for Subesophageal zone-localized Descending GABAergic neurons. The name is based on the structural feature of having neurites that descend from the brain region called the subesophageal zone, which is located in the center of the fly, to the abdominal ganglion (brain region corresponding to the spinal cord in mammals), and on the chemical property of GABA as a neurotransmitter.
Note 4 GABA
Abbreviation for gamma-aminobutyric acid. A typical inhibitory neurotransmitter in Drosophila. Synthesized from glutamate by glutamic acid decarboxylase (Gad1 in Drosophila).
Note5 presynaptic terminal
The site where the tip of an axon in one neuron contacts another neuron to form a synapse.
Note 6 Insulin-like peptide
A type of peptide hormone that is synthesized as a precursor of about 100 amino acids and then cleaved into fragments of lower molecular weight. They show a highly conserved three-dimensional structure from flies to humans, and are responsible for metabolic regulation in response to nutritional conditions such as cell growth and blood glucose control.


