Autism Spectrum Disorder (ASD) is among the most common developmental brain disorders with a prevalence of 1 in 54 children in the US according to 2020 data from the Centers for Disease Control and Prevention. ASD is also one of the most mysterious brain conditions: hundreds of risk genes have been implicated but their pathobiological mechanisms remain poorly understood. Nevertheless, in the last decade research progress based on the analysis of single ASD risk genes in mouse models has yielded insights on how these genes might alter neuronal physiology and behavior. Now, a Japanese research team has identified a synaptic mechanism in mouse that is shared by several ASD risk genes that may enable a scientific framework for one of the major overt symptoms of the disease, social impairment. Reduced expression of either of the genes, CNTNAP2 or AHI1, causes a marked reduction in synaptic communication in a unique brain location, layer II/III pyramidal neurons of prefrontal cortex (PFC), resulting in social behavior and vocalization deficits reminiscent of human symptoms. The findings were published online on October 12 in the journal Nature Communications.
The research team, based at the International Research Center for Neurointelligence (IRCN) and Graduate School of Medicine at The University of Tokyo, used a method called in utero electroporation to reduce gene expression levels of CNTNAP2 (contactin associated protein-like 2) and AHI1 (Abelson helper integration site-1) in layer II/III pyramidal neurons of prefrontal cortex. Using whole cell patch clamp recordings, they discovered a reduction in excitatory synaptic transmission in neurons from both mutant mice. In behavioral tests, both mice showed impairments in social behavior and ultrasonic vocalization similar to reduced social and communication abilities observed in ASD patients. Remarkably, social deficits in both mutant mice could be restored to normal levels with the infusion of a drug called an ampakine (CX546) that enhances excitatory synaptic transmission. This rescue experiment suggests that synaptic transmission in PFC LII/III neuron defects may represent a common mechanism for social deficits in mutant mice and potentially also in patients, and that drugs such as CX546 may be candidate treatments although this remains to be tested in the clinic.
Correspondent: Charles Yokoyama, Ph.D., IRCN Science Writing Core
Reference: Sacai, H., Sakoori, K., Konno, K., Nagahama, K., Honoka, S., Watanabe, T., Watanabe, M., Uesaka, N., Kano, M. (2020) Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nature Communications, published online: October 12, 2020, DOI:10.1038/s41467-020-18861-3
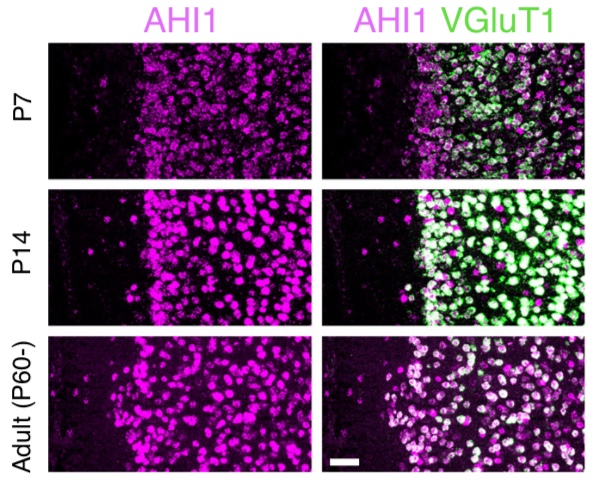
Figure: In situ hybridization immunofluorescence of mRNA for AHI1 and double staining with VGluT1 in cortical layer II/III pyramidal neurons at several developmental time points.
Media Contact: The author is available for interviews in English.
Professor Masanobu Kano, M.D., Ph.D.
International Research Center for Neurointelligence (IRCN)
The University of Tokyo Institutes for Advanced Study
Mayuki Satake
Public Relations
International Research Center for Neurointelligence (IRCN)
The University of Tokyo Institutes for Advanced Study
pr@ircn.jp


