Calcium sensors are among the most effective tools to examine how living neurons behave. Genetically encoded calcium indicators (GECIs) are one type of sensor that when combined with live imaging techniques have become a key tool to understand dynamic brain information processing. Recently, scientists have been developing many types of GECIs to enable long-term, repetitive, and unbiased functional imaging. However, there remain limitations to the GECI toolbox such as the lack of other colors beyond red and green to allow multiplex imaging in adjacent cells, or the inability to accurately record fast spike rates from specific neurons.
Engineering Challenges
A research team led by Professor Haruhiko Bito of the Graduate School of Medicine and the International Research Center for Neurointelligence at The University of Tokyo, set out to solve these issues. A new research study published in the journal Cell unveils new genetic engineering methods for GECIs creating a suite of four color sensors called XCaMPs that enable the measurement of fast brain information processing in vivo. To achieve this resolution, Bito and his colleagues genetically modified existing red and green sensors to create several faster-responding mutants and color-shifted variants.
Variants for Fast or Deep Non-invasive Recordings
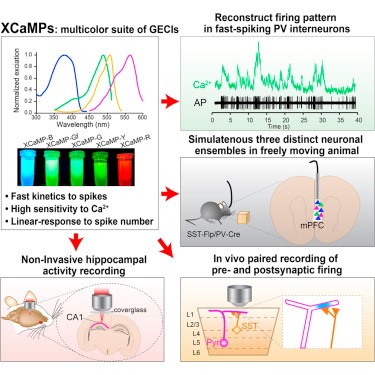
The team made careful measurements of the performance of their multicolor GECIs. They used high-speed line-scan, two-photon excitation microscopy to test the speed performance of the new green indicators revealing ultrafast spiking detection where their responses changed in linear correlation with spike number. The yellow, blue, and red variants also facilitated sensitive detection of Ca2+ rises. In fact, with the application of high-speed imaging techniques to measure neuronal population activity, the green GECI XCaMP-Gf was fast enough to record neural activity from individual fast-spiking neurons and could detect single spikes within 3 to 10 milliseconds of their onset, while the red GECI XCaMP-R enabled non-invasive recording of deep CA1 hippocampal neurons.
Multicolor Circuit Mapping
The spectral diversity of XCaMPs (XCaMP-B, -G, -Y, -R) were advantageous in multicolor circuit mapping where multiple non-overlapping cells could be labeled and recorded. In the medial prefrontal cortex, they could record three distinct (2 inhibitory and 1 excitatory) ensembles in vivo during premotor activity in freely moving mice during investigative behavior. Likewise, paired recordings of pre- and postsynaptic firing tracked axo-dendritic interactions of superficial layer inhibitory interneurons onto apical tuft dendrites of deep layer excitatory neurons. These studies portend exciting new applications in the circuit mapping of complex dynamic neuronal activity.
Future Applications
Bito expects that the newly reported XCaMPs and potentially future variants should benefit neuroscience. The hyperfast suite of four color calcium sensors are suitable for a wide variety of multispectral imaging studies and are particularly compatible with recently developed optical microscopes for the fast readout of neuronal network activity.

“One of the best prospects of XCaMPs are in making precise single cell measurements of how neurons behave”, said Bito.“Our findings should have practical applications because even labs with modest budgets can now hope to achieve fairly sophisticated experiments and perhaps discover new solutions to old problems.
Correspondents: Pham Bich Diem, M.S. and Charles Yokoyama, Ph.D., IRCN Science Writing Core
Reference: Inoue M, Takeuchi A, Manita S, Horigane SI, Sakamoto M, Kawakami R, Yamaguchi K, Otomo K, Yokoyama H, Kim R, Yokoyama T, Takemoto-Kimura S, Abe M, Okamura M, Kondo Y, Quirin S, Ramakrishnan C, Imamura T, Sakimura K, Nemoto T, Kano M, Fujii H, Deisseroth K, Kitamura K, Bito H. Rational Engineering of XCaMPs, a Multicolor GECI Suite for In Vivo Imaging of Complex Brain Circuit Dynamics. Cell, 2019 May 16;177(5):1346-1360.e24. doi: 10.1016/j.cell.2019.04.007.
Graphic Abstract: (reprinted from the original article linked above)
Video Abstract: https://www.youtube.com/watch?v=BoyGNBqM9mg
Media Contact: The author is available for interviews in English.
Professor Haruhiko Bito, M.D., Ph.D.
Chair, Department of Neurochemistry, Graduate School of Medicine
Affiliate Investigator, International Research Center for Neurointelligence
The University of Tokyo
Mayuki Satake
Public Relations
International Research Center for Neurointelligence
The University of Tokyo
pr@ircn.jp


